Conditional survival analysis of patients with resected non-small cell lung cancer
- PMID: 38204712
- PMCID: PMC10775048
- DOI: 10.1016/j.xjon.2023.09.010
Conditional survival analysis of patients with resected non-small cell lung cancer
Abstract
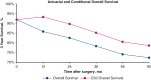
Background: Conditional survival (CS) analyses provide an estimate of survival accounting for years already survived after treatment. We aim to evaluate the difference between actuarial and conditional survival in patients following lung resection for non-small cell lung cancer (NSCLC). In addition, CS analyses are used to examine whether prognosticators of survival change over time following surgery.
Methods: Patients who underwent anatomic lung resection at a single institution for pathologic stage I-IIIA NSCLC between 2010 and 2021 were identified; those who underwent wedge resection for node-negative tumors ≤2 cm were also included. CS estimates were calculated as the probability of remaining disease-free after x years of nonrecurrence (CSx). Kaplan-Meier, log-rank, and Cox proportional hazard methods for examining CS were used for subgroup comparisons and assessing associations with baseline covariates.
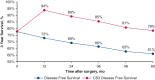
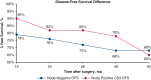
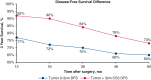
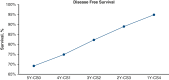
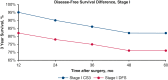
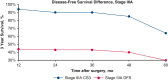
Results: Overall, 863 patients met the study inclusion criteria, with a median follow-up of 44.1 months. Conditional overall survival (OS) and disease-free survival (DFS) were greater than actuarial rates at all time points after surgery. At the time of resection, male sex (hazard ratio [HR], 1.33; 95% confidence interval [CI], 1.03 to 1.72; P = .032), tumor size >3 cm (HR, 1.17; 95% CI, 1.11-1.23; P < .001), node positivity (HR, 3.31; 95% CI, 2.52-4.33; P < .001), and American Joint Committee on Cancer stage (P < .001) were associated with DFS. However, if a patient lived 3 years without recurrence (CS3), these factors were no longer prognostic of DFS.
Conclusions: Conditional survival analyses provide dynamic assessments of OS and DFS after NSCLC resection. After 3 years without recurrence, certain characteristics associated with DFS at the time of surgery no longer prognosticate recurrence.
Keywords: conditional survival; disease-free survival; non–small cell lung cancer; prognosis.
© 2023 The Author(s).
Conflict of interest statement
The authors reported no conflicts of interest. The Journal policy requires editors and reviewers to disclose conflicts of interest and to decline handling or reviewing manuscripts for which they may have a conflict of interest. The editors and reviewers of this article have no conflicts of interest.
Figures






Similar articles
-
The Prognostic Role of PORT and EGFR Mutation Status in Completely Resected Stage IIIA/N2 Non-Small Cell Lung Cancer Patients with Postoperative Chemotherapy.Pathol Oncol Res. 2021 Aug 10;27:1609898. doi: 10.3389/pore.2021.1609898. eCollection 2021. Pathol Oncol Res. 2021. PMID: 34447289 Free PMC article.
-
Changing Odds of Survival Over Time among Patients Undergoing Surgical Resection of Gallbladder Carcinoma.Ann Surg Oncol. 2016 Dec;23(13):4401-4409. doi: 10.1245/s10434-016-5470-2. Epub 2016 Aug 5. Ann Surg Oncol. 2016. PMID: 27495279 Free PMC article.
-
Prognostic Value of Tumor Size in Resected Stage IIIA-N2 Non-Small-Cell Lung Cancer.J Clin Med. 2020 May 1;9(5):1307. doi: 10.3390/jcm9051307. J Clin Med. 2020. PMID: 32370082 Free PMC article.
-
Sublobar resection versus lobectomy for stage IA non-small-cell lung cancer ≤ 2 cm: a systematic review and patient-level meta-analysis.Updates Surg. 2023 Dec;75(8):2343-2354. doi: 10.1007/s13304-023-01627-z. Epub 2023 Aug 10. Updates Surg. 2023. PMID: 37563486
-
Recurrence after surgery in patients with NSCLC.Transl Lung Cancer Res. 2014 Aug;3(4):242-9. doi: 10.3978/j.issn.2218-6751.2013.12.05. Transl Lung Cancer Res. 2014. PMID: 25806307 Free PMC article. Review.
References
-
- Meguid R.A., Hooker C.M., Harris J., Xu L., Westra W.H., Sherwood J.T., et al. Long-term survival outcomes by smoking status in surgical and nonsurgical patients with non-small cell lung cancer: comparing never smokers and current smokers. Chest. 2010;138:500–509. doi: 10.1378/chest.08-2991. - DOI - PMC - PubMed
-
- American Lung Association Key statistics for lung cancer. How common is lung cancer? https://www.cancer.org/cancer/lung-cancer/about/key-statistics.html
-
- Felip E., Altorki N., Zhou C., Csőszi T., Vynnychenko I., Goloborodko O., et al. Adjuvant atezolizumab after adjuvant chemotherapy in resected stage IB–IIIA non-small-cell lung cancer (IMpower010): a randomised, multicentre, open-label, phase 3 trial. Lancet. 2021;398:1344–1357. doi: 10.1016/S0140-6736(21)02098-5. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

