Different Degrees of Sulfated Laminaria Polysaccharides Recovered Damaged HK-2 Cells and Inhibited Adhesion of Nano-COM and Nano-COD Crystals
- PMID: 38204734
- PMCID: PMC10776190
- DOI: 10.1155/2024/8843214
Different Degrees of Sulfated Laminaria Polysaccharides Recovered Damaged HK-2 Cells and Inhibited Adhesion of Nano-COM and Nano-COD Crystals
Abstract
Purpose: The crystal adhesion caused by the damage of renal tubular epithelial cells (HK-2) is the key to the formation of kidney stones. However, no effective preventive drug has been found. This study aims to explore the recovery effects of four Laminaria polysaccharides (SLPs) with different sulfate (-OSO3-) contents on damaged HK-2 cells and the difference in the adhesion of damaged cells to nanometer calcium oxalate monohydrate (COM) and calcium oxalate dihydrate (COD) before and after recovery.
Methods: Sodium oxalate (2.6 mmol/L) was used to damage HK-2 cells to establish a damaged model. SLPs (LP0, SLP1, SLP2, and SLP3) with -OSO3- contents of 0.73%, 15.1%, 22.8%, and 31.3%, respectively, were used to restore the damaged cells, and the effects of SLPs on the adhesion of COM and COD, with a size of about 100 nm before and after recovery, were measured.
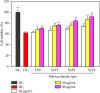
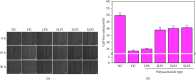
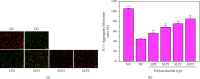
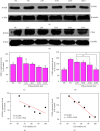
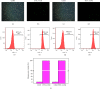
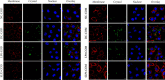
Results: The following results were observed after SLPs recovered the damaged HK-2 cells: increased cell viability, restored cell morphology, decreased reactive oxygen levels, increased mitochondrial membrane potential, decreased phosphatidylserine eversion ratio, increased cell migration ability, reduced expression of annexin A1, transmembrane protein, and heat shock protein 90 on the cell surface, and reduced adhesion amount of cells to COM and COD. Under the same conditions, the adhesion ability of cells to COD crystals was weaker than that to COM crystals.
Conclusions: As the sulfate content in SLPs increases, the ability of SLPs to recover damaged HK-2 cells and inhibit crystal adhesion increases. SLP3 with high -OSO3- content may be a potential drug to prevent kidney stones.
Copyright © 2024 Qiu-Shi Xu et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures






Similar articles
-
Cell Protection and Crystal Endocytosis Inhibition by Sulfated Laminaria Polysaccharides Against Nano-COM-Induced Oxidative Damage in Renal Epithelial Cells.ACS Omega. 2023 Feb 20;8(8):7816-7828. doi: 10.1021/acsomega.2c07584. eCollection 2023 Feb 28. ACS Omega. 2023. PMID: 36872978 Free PMC article.
-
Regulation of Laminaria Polysaccharides with Different Degrees of Sulfation during the Growth of Calcium Oxalate Crystals and their Protective Effects on Renal Epithelial Cells.Oxid Med Cell Longev. 2021 Aug 26;2021:5555796. doi: 10.1155/2021/5555796. eCollection 2021. Oxid Med Cell Longev. 2021. PMID: 34484564 Free PMC article.
-
Sulfated Undaria pinnatifida polysaccharide inhibits the formation of kidney stones by inhibiting HK-2 cell damage and reducing the adhesion of nano‑calcium oxalate crystals.Biomater Adv. 2022 Mar;134:112564. doi: 10.1016/j.msec.2021.112564. Epub 2021 Nov 25. Biomater Adv. 2022. PMID: 35525730
-
Regulatory Effects of Damaged Renal Epithelial Cells After Repair by Porphyra yezoensis Polysaccharides with Different Sulfation Degree on the Calcium Oxalate Crystal-Cell Interaction.Int J Nanomedicine. 2021 Dec 14;16:8087-8102. doi: 10.2147/IJN.S320278. eCollection 2021. Int J Nanomedicine. 2021. PMID: 34934314 Free PMC article.
-
Interaction between nanometer calcium oxalate and renal epithelial cells repaired with carboxymethylated polysaccharides.Biomater Adv. 2022 Jun;137:212854. doi: 10.1016/j.bioadv.2022.212854. Epub 2022 May 13. Biomater Adv. 2022. PMID: 35929244
Cited by
-
Sustainable and biocompatible hybrid materials-based sulfated polysaccharides for biomedical applications: a review.RSC Adv. 2025 Feb 14;15(6):4708-4767. doi: 10.1039/d4ra07277d. eCollection 2025 Feb 6. RSC Adv. 2025. PMID: 39958265 Free PMC article. Review.
References
-
- Guerra A., Ticinesi A., Allegri F., Pinelli S., Aloe R., Meschi T. Idiopathic calcium nephrolithiasis with pure calcium oxalate composition: clinical correlates of the calcium oxalate dihydrate/monohydrate (COD/COM) stone ratio. Urolithiasis . 2020;48(3):271–279. doi: 10.1007/s00240-019-01156-8. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

