Citrus limon (L.) Osbeck Fruit Peel Extract Attenuates Carbon Tetrachloride-Induced Hepatocarcinogenesis in Sprague-Dawley Rats
- PMID: 38204757
- PMCID: PMC10776197
- DOI: 10.1155/2024/6673550
Citrus limon (L.) Osbeck Fruit Peel Extract Attenuates Carbon Tetrachloride-Induced Hepatocarcinogenesis in Sprague-Dawley Rats
Abstract
Background: Traditional herbal medicine practitioners in the Ashanti region of Ghana use the fruit peels of Citrus limon (L.) Osbeck (C. limon) in preventive and curative treatment of many cancers including liver cancer. This ethnobotanical claim remains to be verified scientifically. Aim of the Study. This study investigated prophylactic hepatoprotective and anti-HCC effects of C. limon peel extract (LPE) in CCl4/olive oil-induced HCC-like rats.
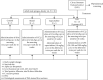
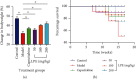
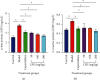
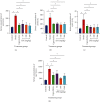
Materials and methods: After preparation of LPE, it was subjected to phytochemical screening using standard phytochemical methods. A total of 30 healthy adult male Sprague-Dawley rats (weighing 150-200 g) were randomly assigned into six groups of 5 rats each. Rats in the control group received olive oil (5 mL/kg ip) twice weekly for 16 weeks. Rats in the model group received CCl4/olive oil (2 mL/kg, ip) twice weekly for 16 weeks. Rats in capecitabine (10 mg/kg po) and LPE (50, 100, and 200 mg/kg po) groups received CCl4/olive oil (2 mL/kg, i.p) in the morning and their respective treatments in the afternoon twice a week for 16 weeks. Rats in all groups had free access to food and water ad libitum. Body weight and survival rates were monitored. Rats were sacrificed under deep anesthesia, blood was collected, and liver and other organs were isolated. Aspartate transaminase (AST), alanine transaminase (ALT), alkaline phosphatase (ALP), gamma-glutamyltransferase (GGT), prothrombin time, bilirubin, C-reactive protein (CRP), alpha- (α-) fetoprotein (AFP), and liver histology were assessed.
Results: Alkaloids, tannins, flavonoids, terpenoids, and saponins were detected in LPE. Model rats demonstrated increased serum levels of AFP, CRP, ALP, GGT, ALT, and AST, prothrombin time, total bilirubin, direct bilirubin, blood lymphocyte, and monocyte counts, but decreased serum albumin and total protein compared to control rats. Unlike the control, model rats demonstrated fat accumulation in periportal and centrilobular hepatocytes and neoplastic transformation. Semiquantitation of periodic acid Schiff- (PAS-) stained liver sections showed decreased glycogen storage in hepatocytes of model rats compared to control rats. Compared to the model, LPE treatment protected against CCl4-induced hepatocarcinogenesis, which was evidenced by decreased AFP, CRP, liver enzymes, total and direct bilirubin, prothrombin time, and blood lymphocyte and monocyte counts; attenuation of fat accumulation; and increased glycogen storage, albumin, and total protein.
Conclusion: LPE abates CCl4-induced hepatocarcinogenesis by attenuating liver inflammation and improving metabolic, biosynthetic, and detoxification functions of the liver. The prophylactic hepatoprotective and anti-hepatocarcinogenic effects of LPE are attributable to its phytochemical composition raising hopes of finding potential anticancer bioactive compounds from C. limon fruit peels.
Copyright © 2024 Alex Boye et al.
Conflict of interest statement
The authors declare that they have no known competing financial interest or personal relationships that could have appeared to influence the work reported in this paper.
Figures




Similar articles
-
Zanthoxylum zanthoxyloides (Lam.) B. Zepernick & Timler alkaloidal extract exerts hepatoprotective effects in rats with a CCl4/olive oil-induced hepatocellular carcinoma-like phenotype.J Taibah Univ Med Sci. 2024 Jul 10;19(4):753-765. doi: 10.1016/j.jtumed.2024.06.009. eCollection 2024 Aug. J Taibah Univ Med Sci. 2024. PMID: 39105209 Free PMC article.
-
Liver protection and hemostatic effects of medicinal plant Arnebia euchroma (Royle) I.M.Johnst extract in a rat model.J Ethnopharmacol. 2023 Jan 10;300:115739. doi: 10.1016/j.jep.2022.115739. Epub 2022 Sep 17. J Ethnopharmacol. 2023. PMID: 36126784
-
Hepatoprotective Efficacy of Cichorium intybus L. Extract Against Carbon Tetrachloride-induced Liver Damage in Rats.J Diet Suppl. 2016;13(5):570-84. doi: 10.3109/19390211.2016.1144230. Epub 2016 Feb 25. J Diet Suppl. 2016. PMID: 26913368
-
Chemical Composition of Golden Berry Leaves Against Hepato-renal Fibrosis.J Diet Suppl. 2016;13(4):378-92. doi: 10.3109/19390211.2015.1099584. Epub 2015 Dec 3. J Diet Suppl. 2016. PMID: 26634867
-
Ameliorative effects of Turbinaria ornata extract on hepatocellular carcinoma induced by diethylnitrosamine in-vivo.J Mol Histol. 2024 Dec;55(6):1225-1238. doi: 10.1007/s10735-024-10263-9. Epub 2024 Oct 1. J Mol Histol. 2024. PMID: 39352545
References
-
- Rawat D., Shrivastava S., Naik R. A., Chhonker S. K., Mehrotra A., Koiri R. K. An overview of natural plant products in the treatment of hepatocellular carcinoma. Anti-Cancer Agents in Medicinal Chemistry . 2018;18(13):1838–1859. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

