Good's Buffer Based Highly Biocompatible Ionic Liquid Modified PLGA Nanoparticles for the Selective Uptake in Cancer Cells
- PMID: 38204762
- PMCID: PMC10776129
- DOI: 10.1039/d3qm00787a
Good's Buffer Based Highly Biocompatible Ionic Liquid Modified PLGA Nanoparticles for the Selective Uptake in Cancer Cells
Abstract
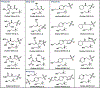
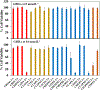
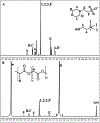
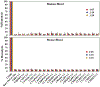
Achieving safe and efficacious drug delivery is still an outstanding challenge. Herein we have synthesized 20 biocompatible Good's buffer-based ionic liquids (GBILs) with a range of attractive properties for drug delivery applications. The synthesized GBILs were used to coat the surface of poly(lactic-co-glycolic acid) (PLGA) by nanoprecipitation-sonication and characterized by dynamic light scattering (DLS) and proton nuclear magnetic resonance (1H NMR) spectroscopy. The GBIL-modified PLGA NPs were then tested for their interaction with bio-interfaces such as serum proteins (using SDS-PAGE and LCMS) and red blood cells (RBCs) isolated from human and BALB/c mouse blood. In this report, we show that surface modification of PLGA with certain GBILs led to modulation of preferential cellular uptake towards human triple-negative breast cancer cells (MDA-MB-231) compared to human normal healthy breast cells (MCF-10A). For example, cholinium N,N-bis(2-hydroxyethyl)-2-aminoethane sulfonate (CBES) coated PLGA NPs were found to be selective for MDA-MB-231 cells (60.7 ± 0.7 %) as compared to MCF-10A cells (27.3 ± 0.7 %). In this way, GBIL-coatings have increased PLGA NP uptake in the cancer cells by 2-fold while decreasing the uptake towards normal healthy breast cells. Therefore, GBIL-modified nanoparticles could be a versatile platform for targeted drug delivery and gene therapy applications, as their surface properties can be tailored to interact with specific cell receptors and enhance cellular uptake. This formulation technique has shown promising results for targeting specific cells, which could be explored further for other cell types to achieve site-specific and efficient delivery of therapeutic agents.
Figures




References
-
- Ferlay J, Colombet M, Soerjomataram I, Parkin DM, Piñeros M, Znaor A and Bray F, Cancer statistics for the year 2020: An overview, 2021, 149, 778–789. - PubMed
-
- Foulkes WD, Smith IE and Reis-Filho JS, Triple-negative breast cancer, N. Engl. J. Med, 2010, 363, 1938–1948. - PubMed
-
- Dropcho EJ, The neurologic side effects of chemotherapeutic agents, CONTIN. Lifelong Learn. Neurol, 2011, 17, 95–112. - PubMed
-
- Love RR, Leventhal H, Easterling DV and Nerenz DRJC, Side effects and emotional distress during cancer chemotherapy, Cancer, 1989, 63, 604–612. - PubMed
-
- Maino DM, Tran S and Mehta F, Side effects of chemotherapeutic oculo-toxic agents: a review, Clin. Eye Vis. Care, 2000, 12, 113–117. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
