The transcription factor VAX1 in VIP neurons of the suprachiasmatic nucleus impacts circadian rhythm generation, depressive-like behavior, and the reproductive axis in a sex-specific manner in mice
- PMID: 38205198
- PMCID: PMC10777845
- DOI: 10.3389/fendo.2023.1269672
The transcription factor VAX1 in VIP neurons of the suprachiasmatic nucleus impacts circadian rhythm generation, depressive-like behavior, and the reproductive axis in a sex-specific manner in mice
Abstract
Background: The suprachiasmatic nucleus (SCN) within the hypothalamus is a key brain structure required to relay light information to the body and synchronize cell and tissue level rhythms and hormone release. Specific subpopulations of SCN neurons, defined by their peptide expression, regulate defined SCN output. Here we focus on the vasoactive intestinal peptide (VIP) expressing neurons of the SCN. SCN VIP neurons are known to regulate circadian rhythms and reproductive function.
Methods: To specifically study SCN VIP neurons, we generated a novel knock out mouse line by conditionally deleting the SCN enriched transcription factor, Ventral Anterior Homeobox 1 (Vax1), in VIP neurons (Vax1Vip; Vax1fl/fl:VipCre).
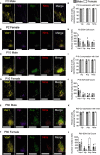
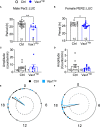
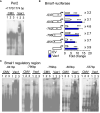
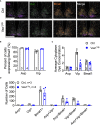
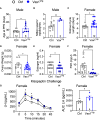
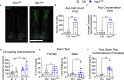
Results: We found that Vax1Vip females presented with lengthened estrous cycles, reduced circulating estrogen, and increased depressive-like behavior. Further, Vax1Vip males and females presented with a shortened circadian period in locomotor activity and ex vivo SCN circadian period. On a molecular level, the shortening of the SCN period was driven, at least partially, by a direct regulatory role of VAX1 on the circadian clock genes Bmal1 and Per2. Interestingly, Vax1Vip females presented with increased expression of arginine vasopressin (Avp) in the paraventricular nucleus, which resulted in increased circulating corticosterone. SCN VIP and AVP neurons regulate the reproductive gonadotropin-releasing hormone (GnRH) and kisspeptin neurons. To determine how the reproductive neuroendocrine network was impacted in Vax1Vip mice, we assessed GnRH sensitivity to a kisspeptin challenge in vivo. We found that GnRH neurons in Vax1Vip females, but not males, had an increased sensitivity to kisspeptin, leading to increased luteinizing hormone release. Interestingly, Vax1Vip males showed a small, but significant increase in total sperm and a modest delay in pubertal onset. Both male and female Vax1Vip mice were fertile and generated litters comparable in size and frequency to controls.
Conclusion: Together, these data identify VAX1 in SCN VIP neurons as a neurological overlap between circadian timekeeping, female reproduction, and depressive-like symptoms in mice, and provide novel insight into the role of SCN VIP neurons.
Keywords: arginine vasopressin; circadian rhythm; cortisol; premenstrual disorders; reproduction; suprachiasmatic nucleus; vasoactive intestinal peptide; ventral anterior homeobox 1.
Copyright © 2023 Van Loh, Yaw, Breuer, Jackson, Nguyen, Jang, Ramos, Ho, Cui, Gillette, Sempere, Gorman, Tonsfeldt, Mellon and Hoffmann.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
The Homeodomain Transcription Factors Vax1 and Six6 Are Required for SCN Development and Function.Mol Neurobiol. 2020 Feb;57(2):1217-1232. doi: 10.1007/s12035-019-01781-9. Epub 2019 Nov 9. Mol Neurobiol. 2020. PMID: 31705443 Free PMC article.
-
The transcription factors SIX3 and VAX1 are required for suprachiasmatic nucleus circadian output and fertility in female mice.J Neurosci Res. 2021 Oct;99(10):2625-2645. doi: 10.1002/jnr.24864. Epub 2021 Jul 2. J Neurosci Res. 2021. PMID: 34212416 Free PMC article.
-
Female fertility does not require Bmal1 in suprachiasmatic nucleus neurons expressing arginine vasopressin, vasoactive intestinal peptide, or neuromedin-S.Front Endocrinol (Lausanne). 2022 Aug 5;13:956169. doi: 10.3389/fendo.2022.956169. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 35992114 Free PMC article.
-
Sex differences in circadian timing systems: implications for disease.Front Neuroendocrinol. 2014 Jan;35(1):111-39. doi: 10.1016/j.yfrne.2013.11.003. Epub 2013 Nov 25. Front Neuroendocrinol. 2014. PMID: 24287074 Free PMC article. Review.
-
An essential role for peptidergic signalling in the control of circadian rhythms in the suprachiasmatic nuclei.J Neuroendocrinol. 2003 Apr;15(4):335-8. doi: 10.1046/j.1365-2826.2003.01005.x. J Neuroendocrinol. 2003. PMID: 12622830 Review.
Cited by
-
Vasoactive intestinal peptide excites GnRH neurons via KCa3.1, a potential player in the slow afterhyperpolarization current.Front Cell Neurosci. 2024 Apr 3;18:1354095. doi: 10.3389/fncel.2024.1354095. eCollection 2024. Front Cell Neurosci. 2024. PMID: 38633445 Free PMC article.
-
Association between bedtime and female infertility: a secondary analysis from a cross-sectional study.Front Endocrinol (Lausanne). 2024 Jun 20;15:1340131. doi: 10.3389/fendo.2024.1340131. eCollection 2024. Front Endocrinol (Lausanne). 2024. PMID: 38966223 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
- R01 HD100580/HD/NICHD NIH HHS/United States
- R25 NS090989/NS/NINDS NIH HHS/United States
- R00 HD084759/HD/NICHD NIH HHS/United States
- T32 HD007203/HD/NICHD NIH HHS/United States
- T32 HD087166/HD/NICHD NIH HHS/United States
- P42 ES010337/ES/NIEHS NIH HHS/United States
- P30 DK063491/DK/NIDDK NIH HHS/United States
- P50 HD028934/HD/NICHD NIH HHS/United States
- K99 NS119291/NS/NINDS NIH HHS/United States
- P30 CA023100/CA/NCI NIH HHS/United States
- T32 GM008666/GM/NIGMS NIH HHS/United States
- F32 HD107852/HD/NICHD NIH HHS/United States
- P50 HD012303/HD/NICHD NIH HHS/United States
- T32 GM007198/GM/NIGMS NIH HHS/United States
- R01 HD072754/HD/NICHD NIH HHS/United States
- R01 HD082567/HD/NICHD NIH HHS/United States
- K99 HD084759/HD/NICHD NIH HHS/United States
- F32 HD090837/HD/NICHD NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

