A glycoengineered therapeutic anti-HBV antibody that allows increased HBsAg immunoclearance improves HBV suppression in vivo
- PMID: 38205373
- PMCID: PMC10777313
- DOI: 10.3389/fphar.2023.1213726
A glycoengineered therapeutic anti-HBV antibody that allows increased HBsAg immunoclearance improves HBV suppression in vivo
Abstract
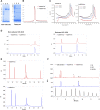
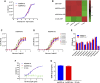
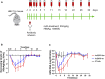
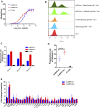
Introduction: The effective and persistent suppression of hepatitis B surface antigen (HBsAg) in patients with chronic HBV infection (CHB) is considered to be a promising approach to achieve a functional cure of hepatitis B. In our previous study, we found that the antibody E6F6 can clear HBsAg through FcγR-mediated phagocytosis, and its humanized form (huE6F6 antibody) is expected to be a new tool for the treatment of CHB. Previous studies have shown that the glycosylation of Fc segments affects the binding of antibodies to FcγR and thus affects the biological activity of antibodies in vivo. Methods: To further improve the therapeutic potential of huE6F6, in this study, we defucosylated huE6F6 (huE6F6-fuc-), preliminarily explored the developability of this molecule, and studied the therapeutic potential of this molecule and its underlying mechanism in vitro and in vivo models. Results: huE6F6-fuc- has desirable physicochemical properties. Compared with huE6F6-wt, huE6F6-fuc- administration resulted in a stronger viral clearance in vivo. Meanwhile, huE6F6-fuc- keep a similar neutralization activity and binding activity to huE6F6-wt in vitro. Immunological analyses suggested that huE6F6-fuc- exhibited enhanced binding to hCD32b and hCD16b, which mainly contributed to its enhanced therapeutic activity in vivo. Conclusions: In summary, the huE6F6-fuc- molecule that was developed in this study, which has desirable developability, can clear HBsAg more efficiently in vivo, providing a promising treatment for CHB patients. Our study provides new guidance for antibody engineering in other disease fields.
Keywords: HBsAg; chronic hepatitis B infection; defucosylation; neutralization; phagocytosis; therapeutic antibody.
Copyright © 2023 You, Chen, Yu, Chen, Wang, Liu, Guo, Zhou, Wang, Zhang, Fang, Zhang, Yue, Wang, Yuan and Luo.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The reviewer (CS) declared a past co-authorship with the authors (WL, YC, XL, CF, XW, BZ, MF, TZ, YQ) to the handling editor
Figures

References
LinkOut - more resources
Full Text Sources

