Improving sub-pixel accuracy in ultrasound localization microscopy using supervised and self-supervised deep learning
- PMID: 38205381
- PMCID: PMC10774911
- DOI: 10.1088/1361-6501/ad1671
Improving sub-pixel accuracy in ultrasound localization microscopy using supervised and self-supervised deep learning
Abstract
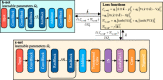
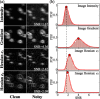
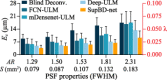
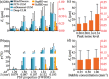
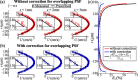
With a spatial resolution of tens of microns, ultrasound localization microscopy (ULM) reconstructs microvascular structures and measures intravascular flows by tracking microbubbles (1-5 μm) in contrast enhanced ultrasound (CEUS) images. Since the size of CEUS bubble traces, e.g. 0.5-1 mm for ultrasound with a wavelength λ = 280 μm, is typically two orders of magnitude larger than the bubble diameter, accurately localizing microbubbles in noisy CEUS data is vital to the fidelity of the ULM results. In this paper, we introduce a residual learning based supervised super-resolution blind deconvolution network (SupBD-net), and a new loss function for a self-supervised blind deconvolution network (SelfBD-net), for detecting bubble centers at a spatial resolution finer than λ/10. Our ultimate purpose is to improve the ability to distinguish closely located microvessels and the accuracy of the velocity profile measurements in macrovessels. Using realistic synthetic data, the performance of these methods is calibrated and compared against several recently introduced deep learning and blind deconvolution techniques. For bubble detection, errors in bubble center location increase with the trace size, noise level, and bubble concentration. For all cases, SupBD-net yields the least error, keeping it below 0.1 λ. For unknown bubble trace morphology, where all the supervised learning methods fail, SelfBD-net can still maintain an error of less than 0.15 λ. SupBD-net also outperforms the other methods in separating closely located bubbles and parallel microvessels. In macrovessels, SupBD-net maintains the least errors in the vessel radius and velocity profile after introducing a procedure that corrects for terminated tracks caused by overlapping traces. Application of these methods is demonstrated by mapping the cerebral microvasculature of a neonatal pig, where neighboring microvessels separated by 0.15 λ can be readily distinguished by SupBD-net and SelfBD-net, but not by the other techniques. Hence, the newly proposed residual learning based methods improve the spatial resolution and accuracy of ULM in micro- and macro-vessels.
Keywords: deep learning; self-supervised learning; super-resolution ultrasound imaging; ultrasound localization microscopy.
© 2024 The Author(s). Published by IOP Publishing Ltd.
Figures


















References
-
- Crapper M, Bruce T, Gouble C. Flow field visualization of sediment-laden flow using ultrasonic imaging. Dyn. Atmos. Oceans. 2000;31:233–45. doi: 10.1016/S0377-0265(99)00035-4. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
