SliceChip: a benchtop fluidic platform for organotypic culture and serial assessment of human and rodent pancreatic slices
- PMID: 38205530
- PMCID: PMC10939771
- DOI: 10.1039/d3lc00850a
SliceChip: a benchtop fluidic platform for organotypic culture and serial assessment of human and rodent pancreatic slices
Abstract
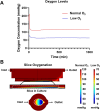
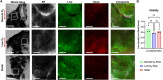
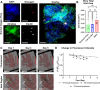
Enzymatically isolated pancreatic islets are the most commonly used ex vivo testbeds for diabetes research. Recently, precision-cut living slices of human pancreas are emerging as an exciting alternative because they maintain the complex architecture of the endocrine and exocrine tissues, and do not suffer from the mechanical and chemical stress of enzymatic isolation. We report a fluidic pancreatic SliceChip platform with dynamic environmental controls that generates a warm, oxygenated, and bubble-free fluidic pathway across singular immobilized slices with continuous deliver of fresh media and the ability to perform repeat serial perfusion assessments. A degasser ensures the system remains bubble-free while systemic pressurization with compressed oxygen ensures slice medium remains adequately oxygenated. Computational modeling of perfusion and oxygen dynamics within SliceChip guide the system's physiomimetic culture conditions. Maintenance of the physiological glucose dependent insulin secretion profile across repeat perfusion assessments of individual pancreatic slices kept under physiological oxygen levels demonstrated the culture capacity of our platform. Fluorescent images acquired every 4 hours of transgenic murine pancreatic slices were reliably stable and recoverable over a 5 day period due to the inclusion of a 3D-printed bioinert metallic anchor that maintained slice position within the SliceChip. Our slice on a chip platform has the potential to expand the useability of human pancreatic slices for diabetes pathogenesis and the development of new therapeutic approaches, while also enabling organotypic culture and assessment of other tissue slices such as brain and patient tumors.
Conflict of interest statement
The authors have no conflicts of interests to disclose.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

