FSH Is Responsible for Androgen Deprivation Therapy-Associated Atherosclerosis in Mice by Exaggerating Endothelial Inflammation and Monocyte Adhesion
- PMID: 38205641
- PMCID: PMC10880942
- DOI: 10.1161/ATVBAHA.123.319426
FSH Is Responsible for Androgen Deprivation Therapy-Associated Atherosclerosis in Mice by Exaggerating Endothelial Inflammation and Monocyte Adhesion
Abstract
Background: Androgen deprivation therapy (ADT) is the mainstay treatment for advanced prostate cancer. But ADTs with orchiectomy and gonadotropin-releasing hormone (GnRH) agonist are associated with increased risk of cardiovascular diseases, which appears less significant with GnRH antagonist. The difference of follicle-stimulating hormone (FSH) in ADT modalities is hypothesized to be responsible for ADT-associated cardiovascular diseases.
Methods: We administered orchiectomy, GnRH agonist, or GnRH antagonist in male ApoE-/- mice fed with Western diet and manipulated FSH levels by testosterone and FSH supplementation or FSH antibody to investigate the role of FSH elevation on atherosclerosis. By combining lipidomics, in vitro study, and intraluminal FSHR (FSH receptor) inhibition, we delineated the effects of FSH on endothelium and monocytes and the underlying mechanisms.
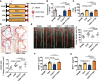
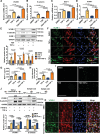
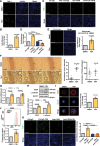
Results: Orchiectomy and GnRH agonist, but not GnRH antagonist, induced long- or short-term FSH elevation and significantly accelerated atherogenesis. In orchiectomized and testosterone-supplemented mice, FSH exposure increased atherosclerosis. In GnRH agonist-treated mice, blocking of short FSH surge by anti-FSHβ antibody greatly alleviated endothelial inflammation and delayed atherogenesis. In GnRH antagonist-treated mice, FSH supplementation aggravated atherogenesis. Mechanistically, FSH, synergizing with TNF-α (tumor necrosis factor alpha), exacerbated endothelial inflammation by elevating VCAM-1 (vascular cell adhesion protein 1) expression through the cAMP/PKA (protein kinase A)/CREB (cAMP response element-binding protein)/c-Jun and PI3K (phosphatidylinositol 3 kinase)/AKT (protein kinase B)/GSK-3β (glycogen synthase kinase 3 beta)/GATA-6 (GATA-binding protein 6) pathways. In monocytes, FSH upregulated CD29 (cluster of differentiation 29) expression via the PI3K/AKT/GSK-3β/SP1 (specificity protein 1) pathway and promoted monocyte-endothelial adhesion both in vitro and in vivo. Importantly, FSHR knockdown by shRNA in endothelium of carotid arteries markedly reduced GnRH agonist-induced endothelial inflammation and atherosclerosis in mice.
Conclusions: FSH is responsible for ADT-associated atherosclerosis by exaggerating endothelial inflammation and promoting monocyte-endothelial adhesion.
Keywords: atherosclerosis; endothelial cells; follicle-stimulating hormone; monocytes; prostatic neoplasms.
Conflict of interest statement
Figures





References
-
- Wong MCS, Goggins WB, Wang HHX, Fung FDH, Leung C, Wong SYS, Ng CF, Sung JJY. Global incidence and mortality for prostate cancer: analysis of temporal patterns and trends in 36 countries. Eur Urol. 2016;70:862–874. doi: 10.1016/j.eururo.2016.05.043 - PubMed
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7–30. doi: 10.3322/caac.21590 - PubMed
-
- Shi F, Xia S. Hormonal therapy and overall management of prostate cancer. Chinese J Cancer Biother. 2018;25:23–27. doi: 10.3872/j.issn.1007-385x.2018.01.004
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

