Adaptive phase I-II clinical trial designs identifying optimal biological doses for targeted agents and immunotherapies
- PMID: 38205644
- PMCID: PMC11132954
- DOI: 10.1177/17407745231220661
Adaptive phase I-II clinical trial designs identifying optimal biological doses for targeted agents and immunotherapies
Abstract
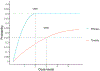
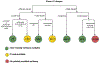
Targeted agents and immunotherapies have revolutionized cancer treatment, offering promising options for various cancer types. Unlike traditional therapies the principle of "more is better" is not always applicable to these new therapies due to their unique biomedical mechanisms. As a result, various phase I-II clinical trial designs have been proposed to identify the optimal biological dose that maximizes the therapeutic effect of targeted therapies and immunotherapies by jointly monitoring both efficacy and toxicity outcomes. This review article examines several innovative phase I-II clinical trial designs that utilize accumulated efficacy and toxicity outcomes to adaptively determine doses for subsequent patients and identify the optimal biological dose, maximizing the overall therapeutic effect. Specifically, we highlight three categories of phase I-II designs: efficacy-driven, utility-based, and designs incorporating multiple efficacy endpoints. For each design, we review the dose-outcome model, the definition of the optimal biological dose, the dose-finding algorithm, and the software for trial implementation. To illustrate the concepts, we also present two real phase I-II trial examples utilizing the EffTox and ISO designs. Finally, we provide a classification tree to summarize the designs discussed in this article.
Keywords: Bayesian adaptive design; Immunotherapy; dose optimization; phase I–II trial; targeted agent.
Conflict of interest statement
Declaration of conflicting interestsThe author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures



References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

