Raludotatug Deruxtecan, a CDH6-Targeting Antibody-Drug Conjugate with a DNA Topoisomerase I Inhibitor DXd, Is Efficacious in Human Ovarian and Kidney Cancer Models
- PMID: 38205802
- PMCID: PMC10911705
- DOI: 10.1158/1535-7163.MCT-23-0287
Raludotatug Deruxtecan, a CDH6-Targeting Antibody-Drug Conjugate with a DNA Topoisomerase I Inhibitor DXd, Is Efficacious in Human Ovarian and Kidney Cancer Models
Abstract
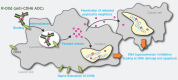
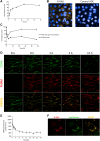
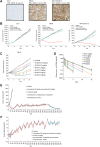
Cadherin-6 (CDH6) is expressed in several cancer types, but no CDH6-targeted therapy is currently clinically available. Here, we generated raludotatug deruxtecan (R-DXd; DS-6000), a novel CDH6-targeting antibody-drug conjugate with a potent DNA topoisomerase I inhibitor, and evaluated its properties, pharmacologic activities, and safety profile. In vitro pharmacologic activities and the mechanisms of action of R-DXd were assessed in serous-type ovarian cancer and renal cell carcinoma cell lines. In vivo pharmacologic activities were evaluated with several human cancer cell lines and patient-derived xenograft mouse models. The safety profile in cynomolgus monkeys was also assessed. R-DXd exhibited CDH6 expression-dependent cell growth-inhibitory activity and induced tumor regression in xenograft models. In this process, R-DXd specifically bound to CDH6, was internalized into cancer cells, and then translocated to the lysosome. The DXd released from R-DXd induced the phosphorylation of Chk1, a DNA damage marker, and cleaved caspase-3, an apoptosis marker, in cancer cells. It was also confirmed that the DXd payload had a bystander effect, passing through the cell membrane and impacting surrounding cells. The safety profile of R-DXd was favorable and the highest non-severely toxic dose was 30 mg/kg in cynomolgus monkeys. R-DXd demonstrated potent antitumor activity against CDH6-expressing tumors in mice and an acceptable safety profile in monkeys. These findings indicate the potential of R-DXd as a new treatment option for patients with CDH6-expressing serous-type ovarian cancer and renal cell carcinoma in a clinical setting.
©2024 The Authors; Published by the American Association for Cancer Research.
Figures



References
-
- Shimoyama Y, Gotoh M, Terasaki T, Kitajima M, Hirohashi S. Isolation and sequence analysis of human cadherin-6 complementary DNA for the full coding sequence and its expression in human carcinoma cells. Cancer Res 1995;55:2206–11. - PubMed
-
- Inoue T, Chisaka O, Matsunami H, Takeichi M. Cadherin-6 expression transiently delineates specific rhombomeres, other neural tube subdivisions, and neural crest subpopulations in mouse embryos. Dev Biol 1997;183:183–94. - PubMed
-
- Cho EA, Patterson LT, Brookhiser WT, Mah S, Kintner C, Dressler GR. Differential expression and function of cadherin-6 during renal epithelium development. Development 1998;125:803–12. - PubMed
-
- Mah SP, Saueressig H, Goulding M, Kintner C, Dressler GR. Kidney development in cadherin-6 mutants: delayed mesenchyme-to-epithelial conversion and loss of nephrons. Dev Biol 2000;223:38–53. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

