Combined immune checkpoint inhibition with durvalumab and tremelimumab with and without radiofrequency ablation in patients with advanced biliary tract carcinoma
- PMID: 38205877
- PMCID: PMC10904979
- DOI: 10.1002/cam4.6912
Combined immune checkpoint inhibition with durvalumab and tremelimumab with and without radiofrequency ablation in patients with advanced biliary tract carcinoma
Abstract
Background: Current standard of care for advanced biliary tract cancer (BTC) is gemcitabine, cisplatin plus anti-PD1/PD-L1, but response rates are modest. The purpose of this study was to explore the efficacy and safety of durvalumab (anti-PD-L1) and tremelimumab (anti-CTLA-4), with and without an interventional radiology (IR) procedure in advanced BTC.
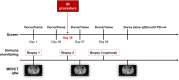
Methods: Eligible patients with advanced BTC who had received or refused at least one prior line of systemic therapy were treated with tremelimumab and durvalumab for four combined doses followed by monthly durvalumab alone with and without an IR procedure until the progression of disease or unacceptable toxicity. Objective response was assessed through CT or MRI by Response Evaluation Criteria in Solid Tumors (RECIST, version 1.1) every 8 weeks. Adverse events (AEs) were recorded and managed. The primary endpoint was 6-month progression-free survival (PFS).
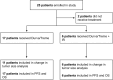
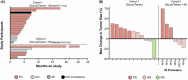
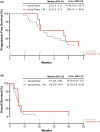
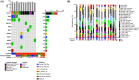
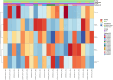
Results: Twenty-three patients with advanced BTC were enrolled; 17 patients were assigned to treatment with durvalumab and tremelimumab (Durva/Treme); and 6 patients were treated with the combination of durvalumab, tremelimumab plus IR procedure (Durva/Treme + IR). The best clinical responses in the Durva/Treme arm were partial response (n = 1), stable disease (n = 5), progressive disease (n = 5), and in the Durva/Treme + IR arm: partial response (n = 0), stable disease (n = 3), progressive disease (n = 3). The median PFS was 2.2 months (95% CI: 1.3-3.1 months) in the Durva/Treme arm and 2.9 months (95% CI: 1.9-4.7 months) in the Durva/Treme + IR arm (p = 0.27). The median OS was 5.1 months (95% CI: 2.5-6.9 months) in the Durva/Treme arm and 5.8 months (95% CI: 2.9-40.1 months) in the Durva/Treme + IR arm (p = 0.31). The majority of AEs were grades 1-2.
Conclusion: Durva/Treme and Durva/Treme + IR showed similar efficacy. With a manageable safety profile. Larger studies are needed to fully characterize the efficacy of Durva/Treme ± IR in advanced BTC.
Trial registration: ClinicalTrials.gov NCT02821754.
Keywords: biliary tract cancer; cholangiocarcinoma; cryoablation; durvalumab; immune checkpoint inhibitor; interventional radiology; radiofrequency ablation; tremelimumab.
Published 2024. This article is a U.S. Government work and is in the public domain in the USA. Cancer Medicine published by John Wiley & Sons Ltd.
Conflict of interest statement
These studies pose no conflicting financial interests for any of the authors.
Figures
References
-
- Bridgewater JA, Goodman KA, Kalyan A, Mulcahy MF. Biliary tract cancer: epidemiology, radiotherapy, and molecular profiling. Am Soc Clin Oncol Educ Book. 2016;35:e194‐e203. - PubMed
-
- Izquierdo‐Sanchez L, Lamarca A, La Casta A, et al. Cholangiocarcinoma landscape in Europe: diagnostic, prognostic and therapeutic insights from the ENSCCA Registry. J Hepatol. 2022;76(5):1109‐1121. - PubMed
-
- Valle JW, Kelley RK, Nervi B, Oh DY, Zhu AX. Biliary tract cancer. Lancet. 2021;397(10272):428‐444. - PubMed
-
- Forner A, Vidili G, Rengo M, Bujanda L, Ponz‐Sarvise M, Lamarca A. Clinical presentation, diagnosis and staging of cholangiocarcinoma. Liver Int. 2019;39(Suppl 1):98‐107. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

