Colony phase variation switch modulates antimicrobial tolerance and biofilm formation in Acinetobacter baumannii
- PMID: 38205963
- PMCID: PMC10845969
- DOI: 10.1128/spectrum.02956-23
Colony phase variation switch modulates antimicrobial tolerance and biofilm formation in Acinetobacter baumannii
Abstract
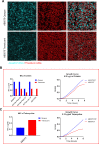
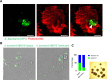
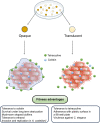
Carbapenem-resistant Acinetobacter baumannii causes one of the most difficult-to-treat nosocomial infections. Polycationic drugs like polymyxin B or colistin and tetracycline drugs such as doxycycline or minocycline are commonly used to treat infections caused by carbapenem-resistant A. baumannii. Here, we show that a subpopulation of cells associated with the opaque/translucent colony phase variation by A. baumannii AB5075 displays differential tolerance to subinhibitory concentrations of colistin and tetracycline. Using a variety of microscopic techniques, we demonstrate that extracellular polysaccharide moieties mediate colistin tolerance to opaque A. baumannii at single-cell level and that mushroom-shaped biofilm structures protect opaque bacteria at the community level. The colony switch phenotype is found to alter several traits of A. baumannii, including long-term survival under desiccation, tolerance to ethanol, competition with Escherichia coli, and intracellular survival in the environmental model host Acanthamoeba castellanii. Additionally, our findings suggest that extracellular DNA associated with membrane vesicles can promote colony switching in a DNA recombinase-dependent manner.IMPORTANCEAs a WHO top-priority drug-resistant microbe, Acinetobacter baumannii significantly contributes to hospital-associated infections worldwide. One particularly intriguing aspect is its ability to reversibly switch its colony morphotype on agar plates, which has been remarkably underexplored. In this study, we employed various microscopic techniques and phenotypic assays to investigate the colony phase variation switch under different clinically and environmentally relevant conditions. Our findings reveal that the presence of a poly N-acetylglucosamine-positive extracellular matrix layer contributes to the protection of bacteria from the bactericidal effects of colistin. Furthermore, we provide intriguing insights into the multicellular lifestyle of A. baumannii, specifically in the context of colony switch variation within its predatory host, Acanthamoeba castellanii.
Keywords: colisitin; opaque colony; translucent colony.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Phenotypic and molecular characterization of Acinetobacter baumannii isolates causing lower respiratory infections among ICU patients.Microb Pathog. 2019 Mar;128:75-81. doi: 10.1016/j.micpath.2018.12.023. Epub 2018 Dec 15. Microb Pathog. 2019. PMID: 30562602
-
Enhancing effect of natural adjuvant, panduratin A, on antibacterial activity of colistin against multidrug-resistant Acinetobacter baumannii.Sci Rep. 2024 Apr 29;14(1):9863. doi: 10.1038/s41598-024-60627-0. Sci Rep. 2024. PMID: 38684853 Free PMC article.
-
In Vitro Interactions of Antibiotic Combinations of Colistin, Tigecycline, and Doripenem Against Extensively Drug-Resistant and Multidrug-Resistant Acinetobacter baumannii.Ann Lab Med. 2016 Mar;36(2):124-30. doi: 10.3343/alm.2016.36.2.124. Ann Lab Med. 2016. PMID: 26709259 Free PMC article.
-
Novel approaches to overcome Colistin resistance in Acinetobacter baumannii: Exploring quorum quenching as a potential solution.Microb Pathog. 2023 Sep;182:106264. doi: 10.1016/j.micpath.2023.106264. Epub 2023 Jul 19. Microb Pathog. 2023. PMID: 37474078 Review.
-
Systematic Review of Antimicrobial Resistance of Clinical Acinetobacter baumannii Isolates in Iran: An Update.Microb Drug Resist. 2017 Sep;23(6):744-756. doi: 10.1089/mdr.2016.0118. Epub 2017 Jan 13. Microb Drug Resist. 2017. PMID: 28085571
Cited by
-
M.marinum lacking epsH shows increased biofilm formation in vitro and boosted antibiotic tolerance in zebrafish.NPJ Biofilms Microbiomes. 2025 Jun 14;11(1):109. doi: 10.1038/s41522-025-00743-5. NPJ Biofilms Microbiomes. 2025. PMID: 40517184 Free PMC article.
-
Phage-encoded depolymerases as a strategy for combating multidrug-resistant Acinetobacter baumannii.Front Cell Infect Microbiol. 2024 Oct 24;14:1462620. doi: 10.3389/fcimb.2024.1462620. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 39512587 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

