Androgen exposure impairs neutrophil maturation and function within the infected kidney
- PMID: 38206009
- PMCID: PMC10865792
- DOI: 10.1128/mbio.03170-23
Androgen exposure impairs neutrophil maturation and function within the infected kidney
Abstract
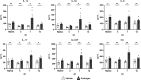
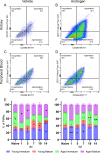
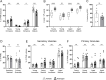
Urinary tract infections (UTIs) in men are uncommon yet carry an increased risk for severe pyelonephritis and other complications. In models of Escherichia coli UTI, C3H/HeN mice develop high-titer pyelonephritis (most with renal abscesses) in a testosterone-dependent manner, but the mechanisms underlying this phenotype are unknown. Here, using female mouse models, we show that androgen exposure impairs neutrophil maturation in the upper and lower urinary tract, compounded by a reduction of neutrophil function within the infected kidney, enabling persistent high-titer infection and promoting abscess formation. Following intravesical inoculation with uropathogenic E. coli (UPEC), kidneys of androgen-exposed C3H mice showed delayed local pro-inflammatory cytokine responses while robustly recruiting neutrophils. These were enriched for an end-organ-specific population of aged but immature neutrophils (CD49d+, CD101-). Compared to their mature counterparts, these aged immature kidney neutrophils exhibited reduced function in vitro, including impaired degranulation and diminished phagocytic activity, while splenic, bone marrow, and bladder neutrophils did not display these alterations. Furthermore, aged immature neutrophils manifested little phagocytic activity within intratubular UPEC communities in vivo. Experiments with B6 conditional androgen receptor (AR)-deficient mice indicated rescue of the maturation defect when AR was deleted in myeloid cells. We conclude that the recognized enhancement of UTI severity by androgens is attributable, at least in part, to local impairment of neutrophil maturation in the urinary tract (largely via cell-intrinsic AR signaling) and a kidney-specific reduction in neutrophil antimicrobial capacity.IMPORTANCEAlthough urinary tract infections (UTIs) predominantly occur in women, male UTIs carry an increased risk of morbidity and mortality. Pyelonephritis in androgen-exposed mice features robust neutrophil recruitment and abscess formation, while bacterial load remains consistently high. Here, we demonstrate that during UTI, neutrophils infiltrating the urinary tract of androgen-exposed mice exhibit reduced maturation, and those that have infiltrated the kidney have reduced phagocytic and degranulation functions, limiting their ability to effectively control infection. This work helps to elucidate mechanisms by which androgens enhance UTI susceptibility and severity, illuminating why male patients may be predisposed to severe outcomes of pyelonephritis.
Keywords: Escherichia coli; immune response; neutrophils; pyelonephritis; sex differences; urinary tract infection.
Conflict of interest statement
D.A.H. serves on the Board of Directors of BioVersys AG, Basel, Switzerland, and has received research funding from BioAge Labs, Richmond, CA, USA. The other authors have no interests to declare.
Figures




Similar articles
-
Androgen exposure potentiates formation of intratubular communities and renal abscesses by Escherichia coli.Kidney Int. 2018 Sep;94(3):502-513. doi: 10.1016/j.kint.2018.04.023. Epub 2018 Jul 3. Kidney Int. 2018. PMID: 30041870 Free PMC article.
-
Androgen-Influenced Polarization of Activin A-Producing Macrophages Accompanies Post-pyelonephritic Renal Scarring.Front Immunol. 2020 Jul 28;11:1641. doi: 10.3389/fimmu.2020.01641. eCollection 2020. Front Immunol. 2020. PMID: 32849562 Free PMC article.
-
Androgens Enhance Male Urinary Tract Infection Severity in a New Model.J Am Soc Nephrol. 2016 Jun;27(6):1625-34. doi: 10.1681/ASN.2015030327. Epub 2015 Oct 8. J Am Soc Nephrol. 2016. PMID: 26449605 Free PMC article.
-
Antibiotic Resistance Among Uropathogenic Escherichia coli.Pol J Microbiol. 2019 Dec;68(4):403-415. doi: 10.33073/pjm-2019-048. Epub 2019 Dec 5. Pol J Microbiol. 2019. PMID: 31880885 Free PMC article. Review.
-
Virulence factors of uropathogenic E. coli and their interaction with the host.Adv Microb Physiol. 2014;65:337-72. doi: 10.1016/bs.ampbs.2014.08.006. Epub 2014 Nov 4. Adv Microb Physiol. 2014. PMID: 25476769 Review.
Cited by
-
Neuroimmune modulation in liver pathophysiology.J Neuroinflammation. 2024 Aug 1;21(1):188. doi: 10.1186/s12974-024-03181-w. J Neuroinflammation. 2024. PMID: 39090741 Free PMC article. Review.
-
Sex Differences in Immune Responses to Infectious Diseases: The Role of Genetics, Hormones, and Aging.Diseases. 2025 Jun 7;13(6):179. doi: 10.3390/diseases13060179. Diseases. 2025. PMID: 40558589 Free PMC article. Review.
-
Testosterone increases the virulence traits of uropathogenic Escherichia coli.Front Microbiol. 2024 May 28;15:1422747. doi: 10.3389/fmicb.2024.1422747. eCollection 2024. Front Microbiol. 2024. PMID: 38863749 Free PMC article.
-
Novel and simplified model for the precise identification of concurrent bacterial infections in patients aged 60 years and older with acute-on-chronic liver diseases: a nationwide, multicentre, prospective cohort study.Hepatol Int. 2025 Jul 28. doi: 10.1007/s12072-025-10855-x. Online ahead of print. Hepatol Int. 2025. PMID: 40719973
-
Sex bias in tumor immunity: insights from immune cells.Theranostics. 2025 Mar 31;15(11):5045-5072. doi: 10.7150/thno.106465. eCollection 2025. Theranostics. 2025. PMID: 40303343 Free PMC article. Review.
References
-
- Ruben FL, Dearwater SR, Norden CW, Kuller LH, Gartner K, Shalley A, Warshafsky G, Kelsey SF, O’Donnell C, Means E, Amoroso W, Bochicchio G. 1995. Clinical infections in the noninstitutionalized geriatric age group: methods utilized and incidence of infections: the Pittsburgh good health study. Am J Epidemiol 141:145–157. doi:10.1093/oxfordjournals.aje.a117402 - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

