Vivaria housing conditions expose sex differences in brain oxidation, microglial activation, and immune system states in aged hAPOE4 mice
- PMID: 38206365
- PMCID: PMC10894770
- DOI: 10.1007/s00221-023-06763-x
Vivaria housing conditions expose sex differences in brain oxidation, microglial activation, and immune system states in aged hAPOE4 mice
Abstract
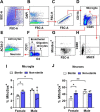
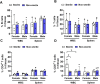
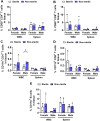
Apolipoprotein E ε4 allele (APOE4) is the predominant genetic risk factor for late-onset Alzheimer's disease (AD). APOE4 mouse models have provided advances in the understanding of disease pathogenesis, but unaccounted variables like rodent housing status may hinder translational outcomes. Non-sterile aspects like food and bedding can be major sources of changes in rodent microflora. Alterations in intestinal microbial ecology can cause mucosal barrier impairment and increase pro-inflammatory signals. The present study examined the role of sterile and non-sterile food and housing on redox indicators and the immune status of humanized-APOE4 knock-in mice (hAPOe4). hAPOE4 mice were housed under sterile conditions until 22 months of age, followed by the transfer of a cohort of mice to non-sterile housing for 2 months. At 24 months of age, the redox/immunologic status was evaluated by flow cytometry/ELISA. hAPOE4 females housed under non-sterile conditions exhibited: (1) higher neuronal and microglial oxygen radical production and (2) lower CD68+ microglia (brain) and CD8+ T cells (periphery) compared to sterile-housed mice. In contrast, hAPOE4 males in non-sterile housing exhibited: (1) higher MHCII+ microglia and CD11b+CD4+ T cells (brain) and (2) higher CD11b+CD4+ T cells and levels of lipopolysaccharide-binding protein and inflammatory cytokines in the periphery relative to sterile-housed mice. This study demonstrated that sterile vs. non-sterile housing conditions are associated with the activation of redox and immune responses in the brain and periphery in a sex-dependent manner. Therefore, housing status may contribute to variable outcomes in both the brain and periphery.
Keywords: Alzheimer’s disease; Apolipoprotein; Housing and food sterility; Immune status; Sex differences.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests that could have influenced the work presented in this manuscript. Financial interests, professional relationships, or personal connections that might have a potential conflict of interest with the research findings are absent.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

