Bioprospection of Phytotoxic Plant-Derived Eudesmanolides and Guaianolides for the Control of Amaranthus viridis, Echinochloa crus-galli, and Lolium perenne Weeds
- PMID: 38206382
- PMCID: PMC10811690
- DOI: 10.1021/acs.jafc.3c06901
Bioprospection of Phytotoxic Plant-Derived Eudesmanolides and Guaianolides for the Control of Amaranthus viridis, Echinochloa crus-galli, and Lolium perenne Weeds
Abstract
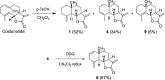
The phytotoxicities of a selection of eudesmanolides and guaianolides, including natural products and new derivatives obtained by semisynthesis from plant-isolated sesquiterpene lactones, were evaluated in bioassays against three weeds of concern in agriculture (Amaranthus viridis L., Echinochloa crus-galli L., and Lolium perenne L.). Both eudesmanolides and guaianolides were active against the root and shoot growth of all the species, with the eudesmanolides generally showing improved activities. The IC50 values obtained for the herbicide employed as positive control (on root and shoot growth, respectively, A. viridis: 27.8 and 85.7 μM; E. crus-galli: 167.5 and 288.2 μM; L. perenne: 99.1 and 571.4 μM) were improved in most of the cases. Structure-activity relationships were discussed, finding that hydroxylation of the A-ring and C-13 as well as the position, number, and orientation of the hydroxyl groups and the presence of an unsaturated carbonyl group can significantly influence the level of phytotoxicity. γ-Cyclocostunolide was the most active compound in the series, followed by others such as dehydrozaluzanin C and α-cyclocostunolide (outstanding their IC50 values on A. viridis)─natural products that can therefore be suggested as models for herbicide development if further research indicates effectiveness on a larger scale and environmental safety in ecotoxicological assessments.
Keywords: bioassays; herbicide development models; phytotoxicity studies; sesquiterpene lactones; structure−activity relationships; weed control.
Conflict of interest statement
The authors declare no competing financial interest.
Figures







Similar articles
-
Evaluation of the phytotoxic and antifungal activity of C17 -sesquiterpenoids as potential biopesticides.Pest Manag Sci. 2022 Oct;78(10):4240-4251. doi: 10.1002/ps.7042. Epub 2022 Jul 8. Pest Manag Sci. 2022. PMID: 35709310 Free PMC article.
-
Structure-activity relationship studies on naphthoquinone analogs. The search for new herbicides based on natural products.Pest Manag Sci. 2019 Sep;75(9):2517-2529. doi: 10.1002/ps.5442. Epub 2019 May 8. Pest Manag Sci. 2019. PMID: 30972945
-
Uptake, translocation, and metabolism of glyphosate, glufosinate, and dicamba mixtures in Echinochloa crus-galli and Amaranthus palmeri.Pest Manag Sci. 2020 Sep;76(9):3078-3087. doi: 10.1002/ps.5859. Epub 2020 May 16. Pest Manag Sci. 2020. PMID: 32281195
-
Phytochemical Study of Safflower Roots (Carthamus tinctorius) on the Induction of Parasitic Plant Germination and Weed Control.J Chem Ecol. 2020 Sep;46(9):871-880. doi: 10.1007/s10886-020-01200-7. Epub 2020 Jul 21. J Chem Ecol. 2020. PMID: 32691372
-
Global perspective of herbicide-resistant weeds.Pest Manag Sci. 2014 Sep;70(9):1306-15. doi: 10.1002/ps.3696. Epub 2014 Jan 15. Pest Manag Sci. 2014. PMID: 24302673 Review.
Cited by
-
Isolation and Identification of Allelopathic Substances from Forsythia suspensa Leaves, and Their Metabolism and Activity.Plants (Basel). 2024 Feb 20;13(5):575. doi: 10.3390/plants13050575. Plants (Basel). 2024. PMID: 38475422 Free PMC article.
-
Phytotoxicity Study of (Amino)imidazo[1,2-a]pyridine Derivatives Toward the Control of Bidens pilosa, Urochloa decumbens, and Panicum maximum Weeds.J Agric Food Chem. 2025 Jan 8;73(1):298-317. doi: 10.1021/acs.jafc.4c09477. Epub 2024 Dec 28. J Agric Food Chem. 2025. PMID: 39731549 Free PMC article.
-
Exploring Sesquiterpene Lactones from Saussurea lappa: Isolation, Structural Modifications, and Herbicide Bioassay Evaluation.Plants (Basel). 2025 Apr 2;14(7):1111. doi: 10.3390/plants14071111. Plants (Basel). 2025. PMID: 40219180 Free PMC article.
References
-
- Dayan F. E.; Duke S. O. Discovery for New Herbicide Sites of Action by Quantification of Plant Primary Metabolite and Enzyme Pools. Engineering 2020, 6 (5), 509–514. 10.1016/j.eng.2020.03.004. - DOI
-
- Castaldi S.; Zorrilla J. G.; Petrillo C.; Russo M. T.; Ambrosino P.; Masi M.; Cimmino A.; Isticato R. Alternaria alternata Isolated from Infected Pears (Pyrus communis) in Italy Produces Non-Host Toxins and Hydrolytic Enzymes as Infection Mechanisms and Exhibits Competitive Exclusion against Botrytis cinerea in Co-Infected Host Fruits. J. Fungi 2023, 9 (3), 326.10.3390/jof9030326. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

