A century of exercise physiology: effects of muscle contraction and exercise on skeletal muscle Na+,K+-ATPase, Na+ and K+ ions, and on plasma K+ concentration-historical developments
- PMID: 38206444
- PMCID: PMC10879387
- DOI: 10.1007/s00421-023-05335-9
A century of exercise physiology: effects of muscle contraction and exercise on skeletal muscle Na+,K+-ATPase, Na+ and K+ ions, and on plasma K+ concentration-historical developments
Abstract
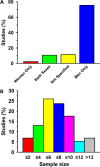
This historical review traces key discoveries regarding K+ and Na+ ions in skeletal muscle at rest and with exercise, including contents and concentrations, Na+,K+-ATPase (NKA) and exercise effects on plasma [K+] in humans. Following initial measures in 1896 of muscle contents in various species, including humans, electrical stimulation of animal muscle showed K+ loss and gains in Na+, Cl- and H20, then subsequently bidirectional muscle K+ and Na+ fluxes. After NKA discovery in 1957, methods were developed to quantify muscle NKA activity via rates of ATP hydrolysis, Na+/K+ radioisotope fluxes, [3H]-ouabain binding and phosphatase activity. Since then, it became clear that NKA plays a central role in Na+/K+ homeostasis and that NKA content and activity are regulated by muscle contractions and numerous hormones. During intense exercise in humans, muscle intracellular [K+] falls by 21 mM (range - 13 to - 39 mM), interstitial [K+] increases to 12-13 mM, and plasma [K+] rises to 6-8 mM, whilst post-exercise plasma [K+] falls rapidly, reflecting increased muscle NKA activity. Contractions were shown to increase NKA activity in proportion to activation frequency in animal intact muscle preparations. In human muscle, [3H]-ouabain-binding content fully quantifies NKA content, whilst the method mainly detects α2 isoforms in rats. Acute or chronic exercise affects human muscle K+, NKA content, activity, isoforms and phospholemman (FXYD1). Numerous hormones, pharmacological and dietary interventions, altered acid-base or redox states, exercise training and physical inactivity modulate plasma [K+] during exercise. Finally, historical research approaches largely excluded female participants and typically used very small sample sizes.
Keywords: Exercise; FXYD; Fatigue; Na+, K+-pump; Plasma; Potassium; Skeletal muscle; Sodium.
© 2024. The Author(s).
Figures





References
-
- Ahlborg B, Bergström J, Ekelund L, Hultman E. Muscle glycogen and muscle electrolytes during prolonged physical exercise. Acta Physiol Scand. 1967;70:129–142. doi: 10.1111/j.1748-1716.1967.tb03608.x. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

