The anterior chamber of the eye technology and its anatomical, optical, and immunological bases
- PMID: 38206586
- PMCID: PMC11381035
- DOI: 10.1152/physrev.00024.2023
The anterior chamber of the eye technology and its anatomical, optical, and immunological bases
Abstract
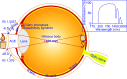
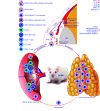
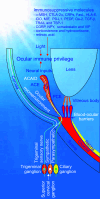
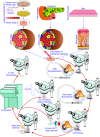
The anterior chamber of the eye (ACE) is distinct in its anatomy, optics, and immunology. This guarantees that the eye perceives visual information in the context of physiology even when encountering adverse incidents like inflammation. In addition, this endows the ACE with the special nursery bed iris enriched in vasculatures and nerves. The ACE constitutes a confined space enclosing an oxygen/nutrient-rich, immune-privileged, and less stressful milieu as well as an optically transparent medium. Therefore, aside from visual perception, the ACE unexpectedly serves as an excellent transplantation site for different body parts and a unique platform for noninvasive, longitudinal, and intravital microimaging of different grafts. On the basis of these merits, the ACE technology has evolved from the prototypical through the conventional to the advanced version. Studies using this technology as a versatile biomedical research platform have led to a diverse range of basic knowledge and in-depth understanding of a variety of cells, tissues, and organs as well as artificial biomaterials, pharmaceuticals, and abiotic substances. Remarkably, the technology turns in vivo dynamic imaging of the morphological characteristics, organotypic features, developmental fates, and specific functions of intracameral grafts into reality under physiological and pathological conditions. Here we review the anatomical, optical, and immunological bases as well as technical details of the ACE technology. Moreover, we discuss major achievements obtained and potential prospective avenues for this technology.
Keywords: in vivo microimaging; innervation; the anterior chamber of the eye; transplantation; vascularization.
Conflict of interest statement
S.-N. Yang is a consultant to Biocrine AB. P.-O. Berggren is the cofounder and CEO of BioCrine AB.
Figures





Similar articles
-
The eye as a novel imaging site in diabetes research.Pharmacol Ther. 2019 May;197:103-121. doi: 10.1016/j.pharmthera.2019.01.005. Epub 2019 Jan 22. Pharmacol Ther. 2019. PMID: 30677477 Free PMC article. Review.
-
Transplantation of tissue grafts into the anterior eye chamber: a method to study intrinsic neurons.Brain Res Brain Res Protoc. 2000 Nov;6(1-2):33-9. doi: 10.1016/s1385-299x(00)00034-9. Brain Res Brain Res Protoc. 2000. PMID: 11086261
-
Intracameral Microimaging of Maturation of Human iPSC Derivatives into Islet Endocrine Cells.Cell Transplant. 2022 Jan-Dec;31:9636897211066508. doi: 10.1177/09636897211066508. Cell Transplant. 2022. PMID: 35156411 Free PMC article.
-
3D-Printed Biohybrid Microstructures Enable Transplantation and Vascularization of Microtissues in the Anterior Chamber of the Eye.Adv Mater. 2024 Jan;36(1):e2306686. doi: 10.1002/adma.202306686. Epub 2023 Oct 18. Adv Mater. 2024. PMID: 37815325
-
Intraocular in vivo imaging of pancreatic islet cell physiology/pathology.Mol Metab. 2017 May 4;6(9):1002-1009. doi: 10.1016/j.molmet.2017.03.014. eCollection 2017 Sep. Mol Metab. 2017. PMID: 28951824 Free PMC article. Review.
Cited by
-
Optogenetics in Pancreatic Islets: Actuators and Effects.Diabetes. 2024 Oct 1;73(10):1566-1582. doi: 10.2337/db23-1022. Diabetes. 2024. PMID: 38976779 Free PMC article. Review.
-
Role of CD4+ T cell-derived cytokines in the pathogenesis of uveitis.Clin Exp Med. 2025 Feb 5;25(1):49. doi: 10.1007/s10238-025-01565-7. Clin Exp Med. 2025. PMID: 39909966 Free PMC article. Review.
References
-
- Shea C, Nemeth SC, DiSclafani M, Allingham RR. Anterior and posterior chambers. In: Ocular Anatomy and Physiology, edited by Lens A, Nemeth SC, Ledford JK.. Thorofare, NJ: SLACK Incorporated, 2008, p. 67–81.
-
- Lens A. Ocular anatomy and physiology jump-start. In: Ocular Anatomy and Physiology, edited by Lens A, Nemeth SC, Ledford JK.. Thorofare, NJ: SLACK Incorporated, 2008, p. 1–6.
-
- van Dooremaal JC. Die entwickelung der in fremden grund versetzten lebenden gewebe. Graefe’s Arhiv Ophthalmol 19: 359–373, 1873. doi:10.1007/BF01693910. - DOI
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

