Accelerated Hypofractionated Chemoradiation Followed by Stereotactic Ablative Radiotherapy Boost for Locally Advanced, Unresectable Non-Small Cell Lung Cancer: A Nonrandomized Controlled Trial
- PMID: 38206614
- PMCID: PMC10784998
- DOI: 10.1001/jamaoncol.2023.6033
Accelerated Hypofractionated Chemoradiation Followed by Stereotactic Ablative Radiotherapy Boost for Locally Advanced, Unresectable Non-Small Cell Lung Cancer: A Nonrandomized Controlled Trial
Abstract
Importance: Intrathoracic progression remains the predominant pattern of failure in patients treated with concurrent chemoradiation followed by a consolidation immune checkpoint inhibitor for locally advanced, unresectable non-small cell lung cancer (NSCLC).
Objective: To determine the maximum tolerated dose (MTD) and use of hypofractionated concurrent chemoradiation with an adaptive stereotactic ablative radiotherapy (SABR) boost.
Design, setting, and participants: This was an early-phase, single-institution, radiation dose-escalation nonrandomized controlled trial with concurrent chemotherapy among patients with clinical stage II (inoperable/patient refusal of surgery) or III NSCLC (American Joint Committee on Cancer Staging Manual, seventh edition). Patients were enrolled and treated from May 2011 to May 2018, with a median patient follow-up of 18.2 months. Patients advanced to a higher SABR boost dose if dose-limiting toxic effects (any grade 3 or higher pulmonary, gastrointestinal, or cardiac toxic effects, or any nonhematologic grade 4 or higher toxic effects) occurred in fewer than 33% of the boost cohort within 90 days of follow-up. The current analyses were conducted from January to September 2023.
Intervention: All patients first received 4 Gy × 10 fractions followed by an adaptive SABR boost to residual metabolically active disease, consisting of an additional 25 Gy (low, 5 Gy × 5 fractions), 30 Gy (intermediate, 6 Gy × 5 fractions), or 35 Gy (high, 7 Gy × 5 fractions) with concurrent weekly carboplatin/paclitaxel.
Main outcome and measure: The primary outcome was to determine the MTD.
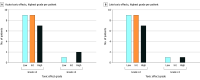
Results: Data from 28 patients (median [range] age, 70 [51-88] years; 16 [57%] male; 24 [86%] with stage III disease) enrolled across the low- (n = 10), intermediate- (n = 9), and high- (n = 9) dose cohorts were evaluated. The protocol-specified MTD was not exceeded. The incidences of nonhematologic acute and late (>90 days) grade 3 or higher toxic effects were 11% and 7%, respectively. No grade 3 toxic effects were observed in the intermediate-dose boost cohort. Two deaths occurred in the high-dose cohort. Two-year local control was 74.1%, 85.7%, and 100.0% for the low-, intermediate-, and high-dose cohorts, respectively. Two-year overall survival was 30.0%, 76.2%, and 55.6% for the low-, intermediate-, and high-dose cohorts, respectively.
Conclusions and relevance: This early-phase, dose-escalation nonrandomized controlled trial showed that concurrent chemoradiation with an adaptive SABR boost to 70 Gy in 15 fractions with concurrent chemotherapy is a safe and effective regimen for patients with locally advanced, unresectable NSCLC.
Trial registration: ClinicalTrials.gov Identifier: NCT01345851.
Conflict of interest statement
Figures


Comment in
-
Stereotactic Body Radiotherapy in Locally Advanced Non-Small Cell Lung Cancer-Is This the Future?JAMA Oncol. 2024 Mar 1;10(3):360-361. doi: 10.1001/jamaoncol.2023.5856. JAMA Oncol. 2024. PMID: 38206616 No abstract available.
-
Locally advanced non-small cell lung cancer: search for the optimal radiotherapy continues.Transl Cancer Res. 2024 Dec 31;13(12):6603-6607. doi: 10.21037/tcr-24-1716. Epub 2024 Dec 26. Transl Cancer Res. 2024. PMID: 39816552 Free PMC article. No abstract available.
-
Accelerated hypofractionated chemoradiation followed by stereotactic ablative radiotherapy boost for locally advanced, unresectable non-small cell lung cancer.Transl Cancer Res. 2025 Feb 28;14(2):676-678. doi: 10.21037/tcr-24-1869. Epub 2025 Feb 26. Transl Cancer Res. 2025. PMID: 40104723 Free PMC article. No abstract available.
Similar articles
-
Accelerated Hypofractionated Radiotherapy for Locally Advanced NSCLC: A Systematic Review From the International Association for the Study of Lung Cancer Advanced Radiation Technology Subcommittee.J Thorac Oncol. 2025 Jan;20(1):39-51. doi: 10.1016/j.jtho.2024.09.1437. Epub 2024 Sep 28. J Thorac Oncol. 2025. PMID: 39349294 Free PMC article.
-
Stereotactic Radiation Therapy in Early Non-Small Cell Lung Cancer and Interstitial Lung Disease: A Nonrandomized Clinical Trial.JAMA Oncol. 2024 May 1;10(5):575-582. doi: 10.1001/jamaoncol.2023.7269. JAMA Oncol. 2024. PMID: 38451491 Free PMC article. Clinical Trial.
-
Concurrent low-dose cisplatin and thoracic radiotherapy in patients with inoperable stage III non-small cell lung cancer: a phase II trial with special reference to the hemoglobin level as prognostic parameter.J Cancer Res Clin Oncol. 2005 Apr;131(4):261-9. doi: 10.1007/s00432-004-0633-0. Epub 2004 Dec 23. J Cancer Res Clin Oncol. 2005. PMID: 15616830 Free PMC article. Clinical Trial.
-
Atezolizumab Before and After Chemoradiation for Unresectable Stage III Non-Small Cell Lung Cancer: A Phase II Nonrandomized Controlled Trial.JAMA Oncol. 2024 Sep 1;10(9):1212-1219. doi: 10.1001/jamaoncol.2024.1897. JAMA Oncol. 2024. PMID: 39052256 Free PMC article. Clinical Trial.
-
Hysterectomy with radiotherapy or chemotherapy or both for women with locally advanced cervical cancer.Cochrane Database Syst Rev. 2015 Apr 7;(4):CD010260. doi: 10.1002/14651858.CD010260.pub2. Cochrane Database Syst Rev. 2015. Update in: Cochrane Database Syst Rev. 2022 Aug 22;8:CD010260. doi: 10.1002/14651858.CD010260.pub3. PMID: 25847525 Updated.
Cited by
-
Innovations in modern low-LET radiotherapy regimens for locally advanced non-small cell lung cancer: a meta-analysis and systematic review of high-dose-rate brachytherapy, stereotactic body radiotherapy, and hypofractionated proton therapy.BMC Cancer. 2025 May 26;25(1):942. doi: 10.1186/s12885-025-14328-0. BMC Cancer. 2025. PMID: 40420058 Free PMC article.
-
A Systematic Review of Phase II/III Trials of Hypofractionated versus Conventionally Fractionated Radiation Therapy in Stage III Non-Small Cell Lung Cancer Patients.Cancers (Basel). 2024 Oct 3;16(19):3384. doi: 10.3390/cancers16193384. Cancers (Basel). 2024. PMID: 39410003 Free PMC article. Review.
-
Lung Stereotactic Body Radiotherapy (SBRT): Challenging Scenarios and New Frontiers.J Clin Med. 2025 Jul 9;14(14):4871. doi: 10.3390/jcm14144871. J Clin Med. 2025. PMID: 40725565 Free PMC article. Review.
-
Accelerated Hypofractionated Radiotherapy for Locally Advanced NSCLC: A Systematic Review From the International Association for the Study of Lung Cancer Advanced Radiation Technology Subcommittee.J Thorac Oncol. 2025 Jan;20(1):39-51. doi: 10.1016/j.jtho.2024.09.1437. Epub 2024 Sep 28. J Thorac Oncol. 2025. PMID: 39349294 Free PMC article.
-
Locally advanced non-small cell lung cancer: search for the optimal radiotherapy continues.Transl Cancer Res. 2024 Dec 31;13(12):6603-6607. doi: 10.21037/tcr-24-1716. Epub 2024 Dec 26. Transl Cancer Res. 2024. PMID: 39816552 Free PMC article. No abstract available.
References
-
- Ahn JS, Ahn YC, Kim JH, et al. . Multinational randomized phase III trial with or without consolidation chemotherapy using docetaxel and cisplatin after concurrent chemoradiation in inoperable stage III non-small-cell lung cancer: KCSG-LU05-04. J Clin Oncol. 2015;33(24):2660-2666. doi:10.1200/JCO.2014.60.0130 - DOI - PubMed
-
- Perez CA, Stanley K, Rubin P, et al. . A prospective randomized study of various irradiation doses and fractionation schedules in the treatment of inoperable non-oat-cell carcinoma of the lung: preliminary report by the Radiation Therapy Oncology Group. Cancer. 1980;45(11):2744-2753. doi:10.1002/1097-0142(19800601)45:11<2744::AID-CNCR2820451108>3.0.CO;2-U - DOI - PubMed
-
- Bradley JD, Paulus R, Komaki R, et al. . Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): a randomised, two-by-two factorial phase 3 study. Lancet Oncol. 2015;16(2):187-199. doi:10.1016/S1470-2045(14)71207-0 - DOI - PMC - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

