Combined Lanreotide Autogel and Temozolomide Treatment of Progressive Pancreatic and Intestinal Neuroendocrine Tumors: The Phase II SONNET Study
- PMID: 38206830
- PMCID: PMC11067796
- DOI: 10.1093/oncolo/oyad325
Combined Lanreotide Autogel and Temozolomide Treatment of Progressive Pancreatic and Intestinal Neuroendocrine Tumors: The Phase II SONNET Study
Abstract
Background: In advanced neuroendocrine tumors (NET), antiproliferative treatment options beyond somatostatin analogs remain limited. Temozolomide (TMZ) has shown efficacy in NET alone or combined with other drugs.
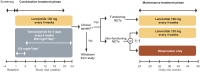
Materials and methods: SONNET (NCT02231762) was an open, multicenter, prospective, phase II study to evaluate lanreotide autogel 120 mg (LAN) plus TMZ in patients with progressive advanced/metastatic grade 1/2 gastroenteropancreatic (GEP) NET or of unknown primary. Patients could be enrolled at first-line or higher therapy line. The primary endpoint was disease control rate ([DCR], rate of stable disease [SD], partial [PR], and complete response [CR]) at 6 months of LAN and TMZ. Patients with nonfunctioning (NF) NET without progression at 6 months were randomized to 6-month LAN maintenance or watch and wait, patients with functioning (F)-NET with clinical benefit (PR, SD) continued on LAN.
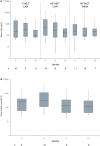
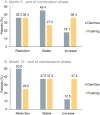
Results: Fifty-seven patients were recruited. The majority of patients received the study drug at second or higher treatment line and had an NET G2. DCR at 6 months LAN and TMZ was 73.5%. After 6 months of further LAN maintenance, 54.5% of patients with F-NET and 71.4% with NF-NET had SD or PR vs 41.7% with NF-NET on observation only. LAN and TMZ were effective in all subgroups analyzed. At 12 months of follow-up, median progression-free survival was 11.1 months. Median serum chromogranin A decreased except in NF-NET on observation. O6-methylguanine DNA methyltransferase promoter methylation appeared to better reflect TMZ response than loss of gene expression. During combination therapy, the most frequent treatment-emergent adverse events grade 3/4 reported were nausea (14%), thrombocytopenia (12.3%), and neutropenia (8.8%). Four deaths were reported resulting from severe adverse events not considered related to study medication.
Conclusions: LAN plus TMZ is a treatment option for patients with progressive GEP-NET with more aggressive biological profile showing a manageable safety profile.
Keywords: O(6)-methylguanine-DNA methyltransferase; gastrointestinal neoplasms; lanreotide; neuroendocrine tumors; receptors; somatostatin; temozolomide.
© The Author(s) 2024. Published by Oxford University Press.
Conflict of interest statement
Marianne Pavel reported a consulting/advisory relationship with Hutchmed and research funding from Ipsen and Novartis as well as honoraria from Ipsen, Novartis, AAA, MSD, Lilly, Boehringer Ingelheim, and streamedup, and Scientific Advisory Board for Ipsen, Novartis, AAA, and Riemser. Harald Lahner reported a consulting/advisory relationship with Ipsen, AAA, and Novartis, and honoraria from Ipsen, AAA, and Novartis. Dieter Hörsch has a consulting/advisory relationship with Ipsen and research funding from Ipsen. . Anja Rinke reported honoraria from Advanz Pharma, Esteve, Ipsen Pharma, and Novartis Pharma (including AAA/ Radiopharmaceuticals) and Scientific Advisory Board for AAA, Advanz Pharma, Esteve, Ipsen, and Novartis. Timm Denecke reported consulting/advisory relationship or honoraria from Bayer, be imaging, MSD, Novartis, Roche, Siemens, Takeda, AAA, AstraZeneca, Ipsen, and Merck, and research funding from Bayer, Siemens, and Guerbet.Arend Koch indicated no financial relationships. Benjamin Regnault is an employee of Ipsen. Dorit Helbig reported employment with Ipsen. Philipp Hoffmanns is an employee of Ipsen. Markus Raderer reported honoraria from Ipsen, Novartis, Celgene, Eli Lilly, Gilead, and Beigene.
Figures

References
-
- Rinke A, Muller HH, Schade-Brittinger C, et al.. Placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: a report from the PROMID Study Group. J Clin Oncol. 2009;27(28):4656-4663. 10.1200/JCO.2009.22.8510 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

