Differential expression and co-localization of transcriptional factors during callus transition to differentiation for shoot organogenesis in the water fern Ceratopteris richardii
- PMID: 38206867
- PMCID: PMC11006541
- DOI: 10.1093/aob/mcae006
Differential expression and co-localization of transcriptional factors during callus transition to differentiation for shoot organogenesis in the water fern Ceratopteris richardii
Abstract
Background and aims: In flowering plants, regeneration can be achieved by a variety of approaches, and different sets of transcriptional factors are involved in these processes. However, regeneration in taxa other than flowering plants remains a mystery. Ceratopteris richardii is a representative fern capable of both direct and indirect organogenesis, and we aimed to investigate the genetics underlying the transition from callus proliferation to differentiation.
Methods: Morphological and histological analyses were used to determine the type of regeneration involved. RNA sequencing and differential gene expression were used to investigate how the callus switches from proliferation to differentiation. Phylogenetic analysis and RNA in situ hybridization were used to understand whether transcriptional factors are involved in this transition.
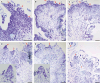
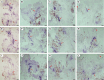
Key results: The callus formed on nascent leaves and subsequently developed the shoot pro-meristem and shoot meristem, thus completing indirect de novo shoot organogenesis in C. richardii. Genes were differentially expressed during the callus transition from proliferation to differentiation, indicating a role for photosynthesis, stimulus response and transmembrane signalling in this transition and the involvement of almost all cell layers that make up the callus. Transcriptional factors were either downregulated or upregulated, which were generally in many-to-many orthology with genes known to be involved in callus development in flowering plants, suggesting that the genetics of fern callus development are both conserved and divergent. Among them, an STM-like, a PLT-like and an ethylene- and salt-inducible ERF gene3-like gene were expressed simultaneously in the vasculature but not in the other parts of the callus, indicating that the vasculature played a role in the callus transition from proliferation to differentiation.
Conclusions: Indirect de novo shoot organogenesis could occur in ferns, and the callus transition from proliferation to differentiation required physiological changes, differential expression of transcriptional factors and involvement of the vasculature.
Keywords: Ceratopteris richardii; in situ hybridization; Transcriptome; callus; ferns; organogenesis; pluripotency; regeneration.
© The Author(s) 2024. Published by Oxford University Press on behalf of the Annals of Botany Company. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Conflict of interest statement
The authors declare they have no conflicts of interest.
Figures




Similar articles
-
Vascular function of the T3/modern clade WUSCHEL-Related HOMEOBOX transcription factor genes predate apical meristem-maintenance function.BMC Plant Biol. 2022 Apr 25;22(1):210. doi: 10.1186/s12870-022-03590-0. BMC Plant Biol. 2022. PMID: 35462532 Free PMC article.
-
Transcriptional analysis of Ceratopteris richardii young sporophyte reveals conservation of stem cell factors in the root apical meristem.Front Plant Sci. 2022 Aug 11;13:924660. doi: 10.3389/fpls.2022.924660. eCollection 2022. Front Plant Sci. 2022. PMID: 36035690 Free PMC article.
-
A De Novo Transcriptome Assembly of Ceratopteris richardii Provides Insights into the Evolutionary Dynamics of Complex Gene Families in Land Plants.Genome Biol Evol. 2021 Mar 1;13(3):evab042. doi: 10.1093/gbe/evab042. Genome Biol Evol. 2021. PMID: 33681974 Free PMC article.
-
KNOX homeobox genes potentially have similar function in both diploid unicellular and multicellular meristems, but not in haploid meristems.Evol Dev. 2005 Jan-Feb;7(1):69-78. doi: 10.1111/j.1525-142X.2005.05008.x. Evol Dev. 2005. PMID: 15642091 Review.
-
Ferns: the missing link in shoot evolution and development.Front Plant Sci. 2015 Nov 6;6:972. doi: 10.3389/fpls.2015.00972. eCollection 2015. Front Plant Sci. 2015. PMID: 26594222 Free PMC article. Review.
Cited by
-
Analysis of auxin responses in the fern Ceratopteris richardii identifies the developmental phase as a major determinant for response properties.Development. 2024 Oct 15;151(20):dev203026. doi: 10.1242/dev.203026. Epub 2024 Sep 26. Development. 2024. PMID: 39324436 Free PMC article.
-
Widespread position-dependent transcriptional regulatory sequences in plants.Nat Genet. 2024 Oct;56(10):2238-2246. doi: 10.1038/s41588-024-01907-3. Epub 2024 Sep 12. Nat Genet. 2024. PMID: 39266765 Free PMC article.
References
-
- Atta R, Laurens L, Boucheron-Dubuisson E, et al. . 2009. Pluripotency of Arabidopsis xylem pericycle underlies shoot regeneration from root and hypocotyl explants grown in vitro. The Plant Journal 57: 626–644. - PubMed
-
- Bui LT, Pandzic D, Youngstrom CE, et al. . 2017. A fern AINTEGUMENTA gene mirrors BABY BOOM in promoting apogamy in Ceratopteris richardii. The Plant Journal 90: 122–132. - PubMed
-
- Cary AJ, Che P, Howell SH.. 2002. Developmental events and shoot apical meristem gene expression patterns during shoot development in Arabidopsis thaliana. The Plant Journal 32: 867–877. - PubMed
-
- Chatfield SP, Capron R, Severino A, et al. . 2013. Incipient stem cell niche conversion in tissue culture: using a systems approach to probe early events in WUSCHEL-dependent conversion of lateral root primordia into shoot meristems. The Plant Journal 73: 798–813. - PubMed
-
- Che P, Lall S, Howell SH.. 2007. Developmental steps in acquiring competence for shoot development in Arabidopsis tissue culture. Planta 226: 1183–1194. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

