Deficiency in astrocyte CCL2 production reduces neuroimmune control of Toxoplasma gondii infection
- PMID: 38206985
- PMCID: PMC10807779
- DOI: 10.1371/journal.ppat.1011710
Deficiency in astrocyte CCL2 production reduces neuroimmune control of Toxoplasma gondii infection
Abstract
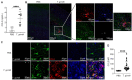
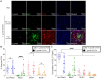
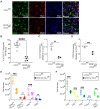
Toxoplasma gondii is an obligate intracellular parasite that infects one-third of the world's human population and establishes infection in the brain. Cerebral immune cell infiltration is critical for controlling the parasite, but little is known about the molecular cues guiding immune cells to the brain during infection. Activated astrocytes produce CCL2, a chemokine that mediates inflammatory monocyte recruitment to tissues by binding to the CCR2 receptor. We detected elevated CCL2 production in the brains of C57BL/6J mice by 15 days after T. gondii infection. Utilizing confocal microscopy and intracellular flow cytometry, we identified microglia and brain-infiltrating myeloid cells as the main producers of CCL2 during acute infection, and CCL2 was specifically produced in regions of parasite infection in the brain. In contrast, astrocytes became the dominant CCL2 producer during chronic T. gondii infection. To determine the role of astrocyte-derived CCL2 in mobilizing immune cells to the brain and controlling T. gondii infection, we generated GFAP-Cre x CCL2fl/fl mice, in which astrocytes are deficient in CCL2 production. We observed significantly decreased immune cell recruitment and increased parasite burden in the brain during chronic, but not acute, infection of mice deficient in astrocyte CCL2 production, without an effect on peripheral immune responses. To investigate potential mechanisms explaining the reduced control of T. gondii infection, we analyzed key antimicrobial and immune players in host defense against T. gondii and detected a reduction in iNOS+ myeloid cells, and T. gondii-specific CD4+ T cells in the knockout mice. These data uncover a critical role for astrocyte-derived CCL2 in immune cell recruitment and parasite control in the brain during chronic, but not acute, T. gondii infection.
Copyright: © 2024 Orchanian et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



Similar articles
-
Astrocytes promote a protective immune response to brain Toxoplasma gondii infection via IL-33-ST2 signaling.PLoS Pathog. 2020 Oct 27;16(10):e1009027. doi: 10.1371/journal.ppat.1009027. eCollection 2020 Oct. PLoS Pathog. 2020. PMID: 33108405 Free PMC article.
-
Alarmin S100A11 initiates a chemokine response to the human pathogen Toxoplasma gondii.Nat Immunol. 2019 Jan;20(1):64-72. doi: 10.1038/s41590-018-0250-8. Epub 2018 Nov 19. Nat Immunol. 2019. PMID: 30455460 Free PMC article.
-
[Rudolf-Virchow Prize 1998. Award lecture. Toxoplasmosis: a model infection for studying systemic and intracerebral immune reactions].Verh Dtsch Ges Pathol. 1998;82:9-22. Verh Dtsch Ges Pathol. 1998. PMID: 10095413 German.
-
Neuroinflammation and schizophrenia: The role of Toxoplasma gondii infection and astrocytic dysfunction.J Neuroimmunol. 2025 Jun 15;403:578588. doi: 10.1016/j.jneuroim.2025.578588. Epub 2025 Mar 19. J Neuroimmunol. 2025. PMID: 40139129 Review.
-
Interferon-gamma- and perforin-mediated immune responses for resistance against Toxoplasma gondii in the brain.Expert Rev Mol Med. 2011 Oct 4;13:e31. doi: 10.1017/S1462399411002018. Expert Rev Mol Med. 2011. PMID: 22005272 Free PMC article. Review.
Cited by
-
Human Retinal Organoid Model of Ocular Toxoplasmosis.Pathogens. 2025 Mar 14;14(3):286. doi: 10.3390/pathogens14030286. Pathogens. 2025. PMID: 40137771 Free PMC article.
-
Targeting Neuroinflammation in Preterm White Matter Injury: Therapeutic Potential of Mesenchymal Stem Cell-Derived Exosomes.Cell Mol Neurobiol. 2025 Mar 12;45(1):23. doi: 10.1007/s10571-025-01540-6. Cell Mol Neurobiol. 2025. PMID: 40072734 Free PMC article. Review.
-
Diverse Subpopulations of Reactive Astrocytes Following Chronic Toxoplasma Infection.Glia. 2025 Oct;73(10):2003-2024. doi: 10.1002/glia.70053. Epub 2025 Jul 9. Glia. 2025. PMID: 40635167 Free PMC article.
-
Protective function and differentiation cues of brain-resident CD8+ T cells during surveillance of latent Toxoplasma gondii infection.Proc Natl Acad Sci U S A. 2024 Jun 11;121(24):e2403054121. doi: 10.1073/pnas.2403054121. Epub 2024 Jun 5. Proc Natl Acad Sci U S A. 2024. PMID: 38838017 Free PMC article.
-
Genetically encoded biosensor for monitoring spatiotemporal dynamics of CCR2 ligands in culture and in vivo.Nat Methods. 2025 Aug;22(8):1731-1741. doi: 10.1038/s41592-025-02742-y. Epub 2025 Jul 15. Nat Methods. 2025. PMID: 40665002
References
-
- Webster JP. Dubey J.P. Toxoplasmosis of Animals and Humans. Parasit Vectors. 2010;3(1):112.
-
- Pappas G, Roussos N, Falagas ME. Toxoplasmosis snapshots: Global status of Toxoplasma gondii seroprevalence and implications for pregnancy and congenital toxoplasmosis. Int J Parasitol. 2009;39(12):1385–94. - PubMed
-
- Hutchinson WM. Experimental Transmission of Toxoplasma gondii. Nature. 1965;206(4987):961–2. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

