Liquid-like condensates mediate competition between actin branching and bundling
- PMID: 38207079
- PMCID: PMC10801869
- DOI: 10.1073/pnas.2309152121
Liquid-like condensates mediate competition between actin branching and bundling
Abstract
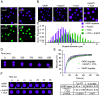
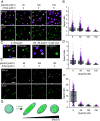
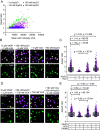
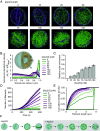
Cellular remodeling of actin networks underlies cell motility during key morphological events, from embryogenesis to metastasis. In these transformations, there is an inherent competition between actin branching and bundling, because steric clashes among branches create a mechanical barrier to bundling. Recently, liquid-like condensates consisting purely of proteins involved in either branching or bundling of the cytoskeleton have been found to catalyze their respective functions. Yet in the cell, proteins that drive branching and bundling are present simultaneously. In this complex environment, which factors determine whether a condensate drives filaments to branch or become bundled? To answer this question, we added the branched actin nucleator, Arp2/3, to condensates composed of VASP, an actin bundling protein. At low actin to VASP ratios, branching activity, mediated by Arp2/3, robustly inhibited VASP-mediated bundling of filaments, in agreement with agent-based simulations. In contrast, as the actin to VASP ratio increased, addition of Arp2/3 led to formation of aster-shaped structures, in which bundled filaments emerged from a branched actin core, analogous to filopodia emerging from a branched lamellipodial network. These results demonstrate that multi-component, liquid-like condensates can modulate the inherent competition between bundled and branched actin morphologies, leading to organized, higher-order structures, similar to those found in motile cells.
Keywords: actin; biomechanics; biomolecular condensate; cytoskeleton; liquid–liquid phase separation.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures

Update of
-
Liquid-like condensates mediate competition between actin branching and bundling.bioRxiv [Preprint]. 2023 Jun 26:2023.06.23.546267. doi: 10.1101/2023.06.23.546267. bioRxiv. 2023. Update in: Proc Natl Acad Sci U S A. 2024 Jan 16;121(3):e2309152121. doi: 10.1073/pnas.2309152121. PMID: 37425724 Free PMC article. Updated. Preprint.
References
-
- Blanchoin L., Boujemaa-Paterski R., Sykes C., Plastino J., Actin dynamics, architecture, and mechanics in cell motility. Physiol. Rev. 94, 235–263 (2014). - PubMed
-
- Mattila P. K., Lappalainen P., Filopodia: Molecular architecture and cellular functions. Nat. Rev. Mol. Cell Biol. 9, 446–454 (2008). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

