Metallomimetic C-F Activation Catalysis by Simple Phosphines
- PMID: 38207215
- PMCID: PMC10811696
- DOI: 10.1021/jacs.3c10614
Metallomimetic C-F Activation Catalysis by Simple Phosphines
Erratum in
-
Addition to "Metallomimetic C-F Activation Catalysis by Simple Phosphines".J Am Chem Soc. 2024 Apr 12;146(16):11578. doi: 10.1021/jacs.4c04291. Online ahead of print. J Am Chem Soc. 2024. PMID: 38606948 Free PMC article. No abstract available.
Abstract
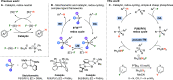
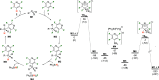
Delivering metallomimetic reactivity from simple p-block compounds is highly desirable in the search to replace expensive, scarce precious metals by cheap and abundant elements in catalysis. This contribution demonstrates that metallomimetic catalysis, involving facile redox cycling between the P(III) and P(V) oxidation states, is possible using only simple, cheap, and readily available trialkylphosphines without the need to enforce unusual geometries at phosphorus or use external oxidizing/reducing agents. Hydrodefluorination and aminodefluorination of a range of fluoroarenes was realized with good to very good yields under mild conditions. Experimental and computational mechanistic studies show that the phosphines undergo oxidative addition of the fluoroaromatic substrate via a Meisenheimer-like transition state to form a fluorophosphorane. This undergoes a pseudotransmetalation step with a silane, via initial fluoride transfer from P to Si, to give experimentally observed phosphonium ions. Hydride transfer from a hydridosilicate counterion then leads to a hydridophosphorane, which undergoes reductive elimination of the product to reform the phosphine catalyst. This behavior is analogous to many classical transition-metal-catalyzed reactions and so is a rare example of both functional and mechanistically metallomimetic behavior in catalysis by a main-group element system. Crucially, the reagents used are cheap, readily available commercially, and easy to handle, making these reactions a realistic prospect in a wide range of academic and industrial settings.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
-
- Weetman C.; Inoue S. The Road Travelled: After Main-Group Elements as Transition Metals. ChemCatChem 2018, 10, 4213–4228. 10.1002/cctc.201800963. - DOI

