Understanding the Termination of Urologic Cancer Clinical Trials: Insights and Challenges
- PMID: 38207249
- PMCID: PMC10793983
- DOI: 10.1200/GO.23.00349
Understanding the Termination of Urologic Cancer Clinical Trials: Insights and Challenges
Abstract
Purpose: Clinical trials are valuable evidence for managing urologic malignancies. Early termination of clinical trials is associated with a waste of resources and may substantially affect patient care. We sought to study the termination rate of urologic cancer clinical trials and identify factors associated with trial termination.
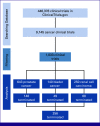
Methods: A cross-sectional search of ClinicalTrials.gov identified completed and terminated kidney, prostate, and bladder cancer clinical trials started. Trials were assessed for reasons for termination. Multivariable analyses were conducted to determine the significant factors associated with the termination.
Results: Between 2000 and 2020, 9,145 oncology clinical trials were conducted, of which 11.30% (n = 1,033) were urologic cancer clinical trials. Of the urologic cancer clinical trials, 25.38% (n = 265) were terminated, with low patient accrual being the most common reason for termination, 52.9% (n = 127). Multivariable analysis showed that only the university funding source odds ratio (OR) of 2.20 (95% CI, 1.45 to 3.32), single-center studies OR of 2.11 (95% CI, 1.59 to 2.81), and sample size of <50 were significant predictors of clinical trial termination OR of 5.26 (95% CI, 3.85 to 7.69); all P values are <.001.
Conclusion: The termination rate of urologic cancer clinical trials was 25%, with low accrual being the most frequently reported reason. Trials funded by a university, single-center trials, and small trials (sample size <50) were associated with early termination. A better understanding of these factors might help researchers, funding agencies, and other stakeholders prioritize resource allocations for multicenter trials that aim to recruit a sufficient number of patients.
Conflict of interest statement
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (
Figures
Similar articles
-
Estimating the rate and reasons of clinical trial failure in urologic oncology.Urol Oncol. 2021 Mar;39(3):154-160. doi: 10.1016/j.urolonc.2020.10.070. Epub 2020 Nov 27. Urol Oncol. 2021. PMID: 33257221 Free PMC article.
-
Early Termination of Oncology Clinical Trials in the United States.Cancer Med. 2023 Mar;12(5):5517-5525. doi: 10.1002/cam4.5385. Epub 2022 Oct 28. Cancer Med. 2023. PMID: 36305832 Free PMC article.
-
Analysis of the Frequency, Characteristics, and Reasons for Termination of Spine-related Clinical Trials.Clin Spine Surg. 2022 Aug 1;35(7):E596-E600. doi: 10.1097/BSD.0000000000001323. Epub 2022 Mar 30. Clin Spine Surg. 2022. PMID: 35351841
-
Analysis of factors leading to early termination in glioblastoma-related clinical trials.J Neurooncol. 2022 Jul;158(3):489-495. doi: 10.1007/s11060-022-04039-y. Epub 2022 Jun 1. J Neurooncol. 2022. PMID: 35648307 Free PMC article. Review.
-
The history and future of the Urologic Oncology Study Group (UOSG) of the Japan Clinical Oncology Group (JCOG).Jpn J Clin Oncol. 2012 May;42(5):363-7. doi: 10.1093/jjco/hys040. Epub 2012 Mar 26. Jpn J Clin Oncol. 2012. PMID: 22450928 Review.
Cited by
-
Early termination and nonpublication of phase III/IV melanoma clinical trials: a cross-sectional study.Proc (Bayl Univ Med Cent). 2024 Dec 18;38(2):179-182. doi: 10.1080/08998280.2024.2439771. eCollection 2025. Proc (Bayl Univ Med Cent). 2024. PMID: 39989995 Free PMC article.

