Sonrotoclax overcomes BCL2 G101V mutation-induced venetoclax resistance in preclinical models of hematologic malignancy
- PMID: 38211332
- PMCID: PMC11076911
- DOI: 10.1182/blood.2023019706
Sonrotoclax overcomes BCL2 G101V mutation-induced venetoclax resistance in preclinical models of hematologic malignancy
Abstract
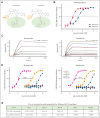
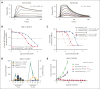
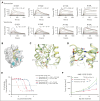
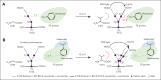
Venetoclax, the first-generation inhibitor of the apoptosis regulator B-cell lymphoma 2 (BCL2), disrupts the interaction between BCL2 and proapoptotic proteins, promoting the apoptosis in malignant cells. Venetoclax is the mainstay of therapy for relapsed chronic lymphocytic leukemia and is under investigation in multiple clinical trials for the treatment of various cancers. Although venetoclax treatment can result in high rates of durable remission, relapse has been widely observed, indicating the emergence of drug resistance. The G101V mutation in BCL2 is frequently observed in patients who relapsed treated with venetoclax and sufficient to confer resistance to venetoclax by interfering with compound binding. Therefore, the development of next-generation BCL2 inhibitors to overcome drug resistance is urgently needed. In this study, we discovered that sonrotoclax, a potent and selective BCL2 inhibitor, demonstrates stronger cytotoxic activity in various hematologic cancer cells and more profound tumor growth inhibition in multiple hematologic tumor models than venetoclax. Notably, sonrotoclax effectively inhibits venetoclax-resistant BCL2 variants, such as G101V. The crystal structures of wild-type BCL2/BCL2 G101V in complex with sonrotoclax revealed that sonrotoclax adopts a novel binding mode within the P2 pocket of BCL2 and could explain why sonrotoclax maintains stronger potency than venetoclax against the G101V mutant. In summary, sonrotoclax emerges as a potential second-generation BCL2 inhibitor for the treatment of hematologic malignancies with the potential to overcome BCL2 mutation-induced venetoclax resistance. Sonrotoclax is currently under investigation in multiple clinical trials.
© 2024 American Society of Hematology. Published by Elsevier Inc. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: All authors are current or previous employees and shareholders of BeiGene (Beijing) Co, Ltd. The design of, conduct of, and financial support for this research were provided by BeiGene (Beijing) Co, Ltd. BeiGene (Beijing) Co, Ltd, participated in data interpretation and the review and approval of the manuscript.
Figures




Comment in
-
BCL2 inhibition: back to the future!Blood. 2024 May 2;143(18):1787-1788. doi: 10.1182/blood.2023023796. Blood. 2024. PMID: 38696193 No abstract available.
References
-
- Tsujimoto Y, Finger LR, Yunis J, Nowell PC, Croce CM. Cloning of the chromosome breakpoint of neoplastic B cells with the t(14;18) chromosome translocation. Science. 1984;226(4678):1097–1099. - PubMed
-
- Hockenbery D, Nuñez G, Milliman C, Schreiber RD, Korsmeyer SJ. Bcl-2 is an inner mitochondrial membrane protein that blocks programmed cell death. Nature. 1990;348(6299):334–336. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

