Breast cancer cells have an increased ferroptosis risk induced by system xc- blockade after deliberately downregulating CYTL1 to mediate malignancy
- PMID: 38211443
- PMCID: PMC10821163
- DOI: 10.1016/j.redox.2024.103034
Breast cancer cells have an increased ferroptosis risk induced by system xc- blockade after deliberately downregulating CYTL1 to mediate malignancy
Abstract
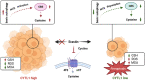
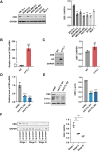
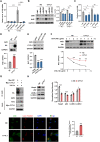
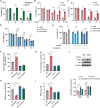
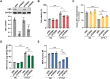
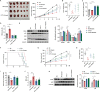
Cytokine-like protein 1 (CYTL1) expression is deliberately downregulated during the progression of multiple types of cancers, especially breast cancer. However, the metabolic characteristics of cancer progression remain unclear. Here, we uncovered a risk of breast cancer cells harboring low CYTL1 expression, which is metabolically controlled during malignant progression. We performed metabolism comparison and revealed that breast cancer cells with low CYTL1 expression have highly suppressed transsulfuration activity that is driven by cystathionine β-synthase (CBS) and contributes to de novo cysteine synthesis. Mechanistically, CYTL1 activated Nrf2 by promoting autophagic Keap1 degradation, and Nrf2 subsequently transactivated CBS expression. Due to the lack of cellular cysteine synthesis, breast cancer cells with low CYTL1 expression showed hypersensitivity to system xc- blockade-induced ferroptosis in vitro and in vivo. Silencing CBS counteracted CYTL1-mediated ferroptosis resistance. Our results show the importance of exogeneous cysteine in breast cancer cells with low CYTL1 expression and highlight a potential metabolic vulnerability to target.
Keywords: Breast cancer; CBS; CYTL1; Ferroptosis; Transsulfuration pathway.
Copyright © 2024 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare no conflict of interest.
Figures

Similar articles
-
Activation of the reverse transsulfuration pathway through NRF2/CBS confers erastin-induced ferroptosis resistance.Br J Cancer. 2020 Jan;122(2):279-292. doi: 10.1038/s41416-019-0660-x. Epub 2019 Dec 10. Br J Cancer. 2020. PMID: 31819185 Free PMC article.
-
Biological insights in non-small cell lung cancer.Cancer Biol Med. 2023 Jun 28;20(7):500-18. doi: 10.20892/j.issn.2095-3941.2023.0108. Cancer Biol Med. 2023. PMID: 37381723 Free PMC article. Review.
-
Apolipoprotein C1 promotes glioblastoma tumorigenesis by reducing KEAP1/NRF2 and CBS-regulated ferroptosis.Acta Pharmacol Sin. 2022 Nov;43(11):2977-2992. doi: 10.1038/s41401-022-00917-3. Epub 2022 May 17. Acta Pharmacol Sin. 2022. PMID: 35581292 Free PMC article.
-
Transsulfuration pathway activation attenuates oxidative stress and ferroptosis in sickle primary erythroblasts and transgenic mice.Commun Biol. 2025 Jan 6;8(1):15. doi: 10.1038/s42003-024-07424-7. Commun Biol. 2025. PMID: 39762627 Free PMC article.
-
The crosstalk between classic cell signaling pathways, non-coding RNAs and ferroptosis in drug resistance of tumors.Cell Signal. 2023 Feb;102:110538. doi: 10.1016/j.cellsig.2022.110538. Epub 2022 Nov 24. Cell Signal. 2023. PMID: 36436800 Review.
Cited by
-
Construction of M2 macrophage-related gene signature for predicting prognosis and revealing different immunotherapy response in bladder cancer patients.Clin Transl Oncol. 2025 May;27(5):2191-2206. doi: 10.1007/s12094-024-03698-9. Epub 2024 Sep 30. Clin Transl Oncol. 2025. PMID: 39347941
-
Multi-omics integration reveals CYTL1 and H6PD as key regulators of tumor metabolism in mesothelioma.Genes Genomics. 2025 Aug 25. doi: 10.1007/s13258-025-01667-2. Online ahead of print. Genes Genomics. 2025. PMID: 40853611
-
Targeting ferroptosis reveals a new strategy for breast cancer treatment: a bibliometric study.Discov Oncol. 2024 Nov 19;15(1):679. doi: 10.1007/s12672-024-01569-x. Discov Oncol. 2024. PMID: 39560863 Free PMC article.
References
-
- Wang Z., Yip L.Y., Lee J.H.J., et al. Methionine is a metabolic dependency of tumor-initiating cells. Nat. Med. 2019;25:825–837. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

