Oral linezolid compared with benzathine penicillin G for treatment of early syphilis in adults (Trep-AB Study) in Spain: a prospective, open-label, non-inferiority, randomised controlled trial
- PMID: 38211601
- PMCID: PMC10954560
- DOI: 10.1016/S1473-3099(23)00683-7
Oral linezolid compared with benzathine penicillin G for treatment of early syphilis in adults (Trep-AB Study) in Spain: a prospective, open-label, non-inferiority, randomised controlled trial
Abstract
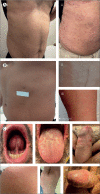
Background: Management of syphilis, a sexually transmitted infection (STI) with increasing incidence, is challenged by drug shortages, scarcity of randomised trial data, an absence of non-penicillin alternatives for pregnant women with penicillin allergy (other than desensitisation), extended parenteral administration for neurosyphilis and congenital syphilis, and macrolide resistance. Linezolid was shown to be active against Treponema pallidum, the causative agent of syphilis, in vitro and in the rabbit model. We aimed to assess the efficacy of linezolid for treating early syphilis in adults compared with the standard of care benzathine penicillin G (BPG).
Methods: We did a multicentre, open-label, non-inferiority, randomised controlled trial to assess the efficacy of linezolid for treating early syphilis compared with BPG. We recruited participants with serological or molecular confirmation of syphilis (either primary, secondary, or early latent) at one STI unit in a public hospital and two STI community clinics in Catalonia (Spain). Participants were randomly allocated in a 1:1 ratio using a computer-generated block randomisation list with six participants per block, to receive either oral linezolid (600 mg once per day for 5 days) or intramuscular BPG (single dose of 2·4 million international units) and were assessed for signs and symptoms (once per week until week 6 and at week 12, week 24, and week 48) and reagin titres of non-treponemal antibodies (week 12, week 24, and week 48). The primary endpoint was treatment response, assessed using a composite endpoint that included clinical response, serological response, and absence of relapse. Clinical response was assessed at 2 weeks for primary syphilis and at 6 weeks for secondary syphilis following treatment initiation. Serological cure was defined as a four-fold decline in rapid plasma reagin titre or seroreversion at any of the 12-week, 24-week, or 48-week timepoints. The absence of relapse was defined as the presence of different molecular sequence types of T pallidum in recurrent syphilis. Non-inferiority was shown if the lower limit of the two-sided 95% CI for the difference in rates of treatment response was higher than -10%. The primary analysis was done in the per-protocol population. The trial is registered at ClinicalTrials.gov (NCT05069974) and was stopped for futility after interim analysis.
Findings: Between Oct 20, 2021, and Sept 15, 2022, 62 patients were assessed for eligibility, and 59 were randomly assigned to linezolid (n=29) or BPG (n=30). In the per-protocol population, after 48 weeks' follow-up, 19 (70%) of 27 participants (95% CI 49·8 to 86·2) in the linezolid group had responded to treatment and 28 (100%) of 28 participants (87·7 to 100·0) in the BPG group (treatment difference -29·6, 95% CI -50·5 to -8·8), which did not meet the non-inferiority criterion. The number of drug-related adverse events (all mild or moderate) was similar in both treatment groups (five [17%] of 29, 95% CI 5·8 to 35·8 in the linezolid group vs five [17%] of 30, 5·6 to 34·7, in the BPG group). No serious adverse events were reported during follow-up.
Interpretation: The efficacy of linezolid at a daily dose of 600 mg for 5 days did not meet the non-inferiority criteria compared with BPG and, as a result, this treatment regimen should not be used to treat patients with early syphilis.
Funding: European Research Council and Fondo de Investigaciones Sanitarias.
Copyright © 2024 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY-NC-ND 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of interests RP received grants or contracts from MSD and ViiV Healthcare (payments to his institution) and consulting fees from Pfizer, Gilead, GSK, AstraZeneca, Atea, and Roche. AC has received payment or honoraria for lectures, presentations, speakers' bureaus, manuscript writing, or educational events from Pfizer, Merck, Biomerieux, Becton, and Seegene, and has received support for attending meetings or travel from Beckman. YH-M has grants or contracts with Seegene (payments to his institution). All other authors declare no competing interests.
Figures

References
-
- Goldenberg RL, Thompson C. The infectious origins of stillbirth. Am J Obstet Gynecol. 2003;189:861–873. - PubMed
-
- Janier M, Unemo M, Dupin N, Tiplica GS, Potočnik M, Patel R. 2020 European guideline on the management of syphilis. J Eur Acad Dermatol Venereol. 2021;35:574–588. - PubMed
-
- WHO Global shortages of penicillin. Feb 9, 2023. https://www.who.int/teams/global-hiv-hepatitis-and-stis-programmes/stis/...
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

