Disruption of lysosomal nutrient sensing scaffold contributes to pathogenesis of a fatal neurodegenerative lysosomal storage disease
- PMID: 38211816
- PMCID: PMC10862020
- DOI: 10.1016/j.jbc.2024.105641
Disruption of lysosomal nutrient sensing scaffold contributes to pathogenesis of a fatal neurodegenerative lysosomal storage disease
Abstract
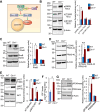
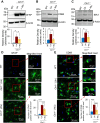
The ceroid lipofuscinosis neuronal 1 (CLN1) disease, formerly called infantile neuronal ceroid lipofuscinosis, is a fatal hereditary neurodegenerative lysosomal storage disorder. This disease is caused by loss-of-function mutations in the CLN1 gene, encoding palmitoyl-protein thioesterase-1 (PPT1). PPT1 catalyzes depalmitoylation of S-palmitoylated proteins for degradation and clearance by lysosomal hydrolases. Numerous proteins, especially in the brain, require dynamic S-palmitoylation (palmitoylation-depalmitoylation cycles) for endosomal trafficking to their destination. While 23 palmitoyl-acyl transferases in the mammalian genome catalyze S-palmitoylation, depalmitoylation is catalyzed by thioesterases such as PPT1. Despite these discoveries, the pathogenic mechanism of CLN1 disease has remained elusive. Here, we report that in the brain of Cln1-/- mice, which mimic CLN1 disease, the mechanistic target of rapamycin complex-1 (mTORC1) kinase is hyperactivated. The activation of mTORC1 by nutrients requires its anchorage to lysosomal limiting membrane by Rag GTPases and Ragulator complex. These proteins form the lysosomal nutrient sensing scaffold to which mTORC1 must attach to activate. We found that in Cln1-/- mice, two constituent proteins of the Ragulator complex (vacuolar (H+)-ATPase and Lamtor1) require dynamic S-palmitoylation for endosomal trafficking to the lysosomal limiting membrane. Intriguingly, Ppt1 deficiency in Cln1-/- mice misrouted these proteins to the plasma membrane disrupting the lysosomal nutrient sensing scaffold. Despite this defect, mTORC1 was hyperactivated via the IGF1/PI3K/Akt-signaling pathway, which suppressed autophagy contributing to neuropathology. Importantly, pharmacological inhibition of PI3K/Akt suppressed mTORC1 activation, restored autophagy, and ameliorated neurodegeneration in Cln1-/- mice. Our findings reveal a previously unrecognized role of Cln1/Ppt1 in regulating mTORC1 activation and suggest that IGF1/PI3K/Akt may be a targetable pathway for CLN1 disease.
Keywords: CLN1 disease; IGF1 signaling; S-palmitoylation; lysosomal storage disease; mTORC1 activation; neurodegeneration.
Published by Elsevier Inc.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflicts of interest with the contents of this article.
Figures





References
-
- Platt F.M. Emptying the stores: lysosomal diseases and therapeutic strategies. Nat. Rev. Drug Discov. 2018;17:133–150. - PubMed
-
- Fraldi A., Klein A.D., Medina D.L., Settembre C. Brain disorders due to lysosomal dysfunction. Annu. Rev. Neurosci. 2016;39:277–295. - PubMed
-
- de Duve C. The lysosome turns fifty. Nat. Cell Biol. 2005;7:847–849. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

