Long-Term Overall Survival After Selective Internal Radiation Therapy for Locally Advanced Hepatocellular Carcinomas: Updated Analysis of DOSISPHERE-01 Trial
- PMID: 38212068
- PMCID: PMC10858378
- DOI: 10.2967/jnumed.123.266211
Long-Term Overall Survival After Selective Internal Radiation Therapy for Locally Advanced Hepatocellular Carcinomas: Updated Analysis of DOSISPHERE-01 Trial
Abstract
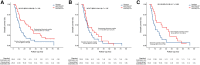
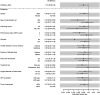
Interim analysis of the DOSISPHERE-01 study demonstrated a strong improvement in response and overall survival (OS) on using 90Y-loaded glass microspheres with personalized dosimetry compared with standard dosimetry in patients with nonoperable locally advanced hepatocellular carcinoma. This report sought to provide a long-term analysis of OS. Methods: In this phase II study (ClinicalTrials.gov identifier NCT02582034), treatment was randomly assigned (1:1) with the goal to deliver either at least 205 Gy (if possible >250-300 Gy) to the index lesion in the personalized dosimetry approach (PDA) or 120 ± 20 Gy to the treated volume in the standard dosimetry approach (SDA). The 3-mo response of the index lesion was the primary endpoint, with OS being one of the secondary endpoints. This report is a post hoc long-term analysis of OS. Results: Overall, 60 hepatocellular carcinoma patients with at least 1 lesion larger than 7 cm and more than 30% of hepatic reserve were randomized (intent-to-treat population: PDA, n = 31; SDA, n = 29), with 56 actually treated (modified intent-to-treat population: n = 28 in each arm). The median follow-up for long-term analysis was 65.8 mo (range, 2.1-73.1 mo). Median OS was 24.8 mo and 10.7 mo (hazard ratio [HR], 0.51; 95% CI, 0.29-0.9; P = 0.02) for PDA and SDA, respectively, in the modified intent-to-treat population. Median OS was 22.9 mo for patients with a tumor dose of at least 205 Gy, versus 10.3 mo for those with a tumor dose of less than 205 Gy (HR, 0.42; 95% CI, 0.22-0.81; P = 0.0095), and was 22.9 mo for patients with a perfused liver dose of 150 Gy or higher, versus 10.3 mo for those with a perfused liver dose of less than 150 Gy (HR, 0.42; 95% CI, 0.23-0.75; P = 0.0033). Lastly, median OS was not reached in patients who were secondarily resected (n = 11, 10 in the PDA group and 1 in the SDA group), versus 10.8 mo in those without secondary resection (n = 45) (HR, 0.17; 95% CI, 0.065-0.43; P = 0.0002). Only resected patients displayed favorable long-term OS rates, meaning an OS of more than 50% at 5 y. Conclusion: After longer follow-up, personalized dosimetry sustained a meaningful improvement in OS, which was dramatically improved for patients who were accurately downstaged toward resection, including most portal vein thrombosis patients.
Keywords: 90Y-loaded glass microspheres; downstaged; hepatocellular carcinoma; personalized dosimetry; portal vein thrombosis.
© 2024 by the Society of Nuclear Medicine and Molecular Imaging.
Figures


Comment in
-
Clinical Outcomes After Personalized Dosimetry for 90Y Radioembolization of Advanced Hepatocellular Carcinoma: Defining the Role of a Device in a Pharma-Centric Landscape.J Nucl Med. 2024 Feb 1;65(2):270-271. doi: 10.2967/jnumed.123.267035. J Nucl Med. 2024. PMID: 38212067 No abstract available.
References
-
- Sung H, Ferlay J, Siegel RL, et al. . Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. - PubMed
-
- Salem R, Gabr A, Riaz A, et al. . Institutional decision to adopt Y90 as primary treatment for hepatocellular carcinoma informed by a 1000-patient 15-year experience. Hepatology. 2018;68:1429–1440. - PubMed
-
- Vilgrain V, Pereira H, Assenat E, et al. . Efficacy and safety of selective internal radiotherapy with yttrium-90 resin microspheres compared with sorafenib in locally advanced and inoperable hepatocellular carcinoma (SARAH): an open-label randomised controlled phase 3 trial. Lancet Oncol. 2017;18:1624–1636. - PubMed
-
- Chow PKH, Gandhi M, Tan S-B, et al. . SIRveNIB: selective internal radiation therapy versus sorafenib in Asia-Pacific patients with hepatocellular carcinoma. J Clin Oncol. 2018;36:1913–1921. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
