Sfrp1 inhibits lung fibroblast invasion during transition to injury-induced myofibroblasts
- PMID: 38212077
- PMCID: PMC10850614
- DOI: 10.1183/13993003.01326-2023
Sfrp1 inhibits lung fibroblast invasion during transition to injury-induced myofibroblasts
Abstract
Background: Fibroblast-to-myofibroblast conversion is a major driver of tissue remodelling in organ fibrosis. Distinct lineages of fibroblasts support homeostatic tissue niche functions, yet their specific activation states and phenotypic trajectories during injury and repair have remained unclear.
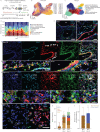
Methods: We combined spatial transcriptomics, multiplexed immunostainings, longitudinal single-cell RNA-sequencing and genetic lineage tracing to study fibroblast fates during mouse lung regeneration. Our findings were validated in idiopathic pulmonary fibrosis patient tissues in situ as well as in cell differentiation and invasion assays using patient lung fibroblasts. Cell differentiation and invasion assays established a function of SFRP1 in regulating human lung fibroblast invasion in response to transforming growth factor (TGF)β1.
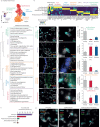
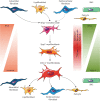
Measurements and main results: We discovered a transitional fibroblast state characterised by high Sfrp1 expression, derived from both Tcf21-Cre lineage positive and negative cells. Sfrp1 + cells appeared early after injury in peribronchiolar, adventitial and alveolar locations and preceded the emergence of myofibroblasts. We identified lineage-specific paracrine signals and inferred converging transcriptional trajectories towards Sfrp1 + transitional fibroblasts and Cthrc1 + myofibroblasts. TGFβ1 downregulated SFRP1 in noninvasive transitional cells and induced their switch to an invasive CTHRC1+ myofibroblast identity. Finally, using loss-of-function studies we showed that SFRP1 modulates TGFβ1-induced fibroblast invasion and RHOA pathway activity.
Conclusions: Our study reveals the convergence of spatially and transcriptionally distinct fibroblast lineages into transcriptionally uniform myofibroblasts and identifies SFRP1 as a modulator of TGFβ1-driven fibroblast phenotypes in fibrogenesis. These findings are relevant in the context of therapeutic interventions that aim at limiting or reversing fibroblast foci formation.
Copyright ©The authors 2024.
Conflict of interest statement
Conflict of interest: M. Gerckens reports grants from Stiftung Atemweg e.V. and a patent pending EP21178481 “Novel anti-fibrotic drugs”, outside the submitted work. M. Tallquist reports support for the present manuscript from NIH (5R21HL156112). J. Beckers reports funding for the current manuscript, as well as funding for consumables outside the submitted work, from Helmholtz Zentrum München GmbH. O. Eickelberg reports support for the present manuscript from R01 HL146519; in addition, O. Eickelberg reports consulting fees from Blade Therapeutics, Yap Therapeutics and Pieris Pharmaceuticals, stock or stock options from Blade Therapeutics, outside the submitted work. J. Behr reports a leadership role as Chair of Guideline Committee of the German Respiratory Society (DGP), outside the submitted work. W.A. Wuyts reports grants, consulting fees, lecture honoraria and advisory board participation from Roche, Pliant, Boehringer Ingelheim, Alentis and Galapagos. K. Ahlbrecht reports support for the present manuscript from Max Planck Society, German Center for Lung Research (Deutsches Zentrum für Lungenforschung; DZL), Federal Ministry of Higher Education, Research and the Arts of the State of Hessen LOEWE, Programme through grant UGMLC; in addition, K. Ahlbrecht reports grants from Rhon Klinikum AG (grant FI_71), outside the submitted work. R.E. Morty reports leadership roles as Editor-in-Chief, American Journal of Physiology – Lung Cellular and Molecular Physiology and Group Chair, Group 07.08 Lung and Airway Development, at the European Respiratory Society, outside the submitted work. C. Samakovlis reports grants from Swedish Research Council, Swedish Cancer Society, DFG, Stockholm University, Stockholm, Sweden, Justus-Liebig University, Giessen, Germany, DiscovAir, EU, payment for expert testimony from Swedish Cancer Society and Wallengberg Foundation, and a leadership role with Royal Academy of Science, Sweden, outside the submitted work. F.J. Theis reports support for the present manuscript from the Chan Zuckerburg Foundation (grant number 2019- 002438), as well as consulting fees from Roche, Immunai, Singularity, Omniscope and CytoReason, lecture honoraria from Genentech Research Organisation, AMGEN GmbH, Munich, Roche Germany, Roche, Basel, ETH Zurich, Vizgen, ThirdRockVentures and Pfizer, advisory board participation with Max Planck Institute for Intelligent Systems, Berlin Institute of Health and EMBL, and stock or stock options from Cellarity, outside the submitted work. H.B. Schiller reports support for the present manuscript from Helmholtz Association, Deutsches Zentrum für Lungenforschung (DZL) and CZI/H2020 (discovair). The remaining authors have no potential conflicts of interest to disclose.
Figures





Comment in
-
Fibroblast heterogeneity in pulmonary fibrosis: a new target for therapeutics development?Eur Respir J. 2024 Feb 8;63(2):2302188. doi: 10.1183/13993003.02188-2023. Print 2024 Feb. Eur Respir J. 2024. PMID: 38331439 No abstract available.
-
The concept of Sfrp1+ transitional fibroblasts: the key to dissociating lineage heterogeneity and fate of invasive fibroblasts in pulmonary fibrosis?Eur Respir J. 2024 May 9;63(5):2400498. doi: 10.1183/13993003.00498-2024. Print 2024 May. Eur Respir J. 2024. PMID: 38724178 Free PMC article.
Similar articles
-
CTHRC1: An Emerging Hallmark of Pathogenic Fibroblasts in Lung Fibrosis.Cells. 2024 May 30;13(11):946. doi: 10.3390/cells13110946. Cells. 2024. PMID: 38891078 Free PMC article. Review.
-
Highlighting fibroblast plasticity in lung fibrosis: the WI-38 cell line as a model for investigating the myofibroblast and lipofibroblast switch.Theranostics. 2024 Jun 11;14(9):3603-3622. doi: 10.7150/thno.93519. eCollection 2024. Theranostics. 2024. PMID: 38948058 Free PMC article.
-
Transforming growth factor β1 (TGFβ1)-induced CD44V6-NOX4 signaling in pathogenesis of idiopathic pulmonary fibrosis.J Biol Chem. 2017 Jun 23;292(25):10490-10519. doi: 10.1074/jbc.M116.752469. Epub 2017 Apr 7. J Biol Chem. 2017. PMID: 28389561 Free PMC article.
-
Sirtuin 6 inhibits myofibroblast differentiation via inactivating transforming growth factor-β1/Smad2 and nuclear factor-κB signaling pathways in human fetal lung fibroblasts.J Cell Biochem. 2019 Jan;120(1):93-104. doi: 10.1002/jcb.27128. Epub 2018 Sep 19. J Cell Biochem. 2019. PMID: 30230565
-
Matrix abnormalities in pulmonary fibrosis.Eur Respir Rev. 2018 Jun 27;27(148):180033. doi: 10.1183/16000617.0033-2018. Print 2018 Jun 30. Eur Respir Rev. 2018. PMID: 29950306 Free PMC article. Review.
Cited by
-
PIEZO1 mediates periostin+ myofibroblast activation and pulmonary fibrosis in mice.J Clin Invest. 2025 Jun 2;135(11):e184158. doi: 10.1172/JCI184158. eCollection 2025 Jun 2. J Clin Invest. 2025. PMID: 40454481 Free PMC article.
-
Transcriptome Analysis of Fibroblasts in Hypoxia-Induced Vascular Remodeling: Functional Roles of CD26/DPP4.Int J Mol Sci. 2024 Nov 23;25(23):12599. doi: 10.3390/ijms252312599. Int J Mol Sci. 2024. PMID: 39684311 Free PMC article.
-
Triggering AHR resolves TGF-β1 induced fibroblast activation and promotes AT1 cell regeneration in alveolar organoids.Commun Biol. 2025 Jul 9;8(1):1025. doi: 10.1038/s42003-025-08446-5. Commun Biol. 2025. PMID: 40634525 Free PMC article.
-
PGF2α signaling drives fibrotic remodeling and fibroblast population dynamics in mice.JCI Insight. 2023 Dec 22;8(24):e172977. doi: 10.1172/jci.insight.172977. JCI Insight. 2023. PMID: 37934604 Free PMC article.
-
CTHRC1: An Emerging Hallmark of Pathogenic Fibroblasts in Lung Fibrosis.Cells. 2024 May 30;13(11):946. doi: 10.3390/cells13110946. Cells. 2024. PMID: 38891078 Free PMC article. Review.
References
-
- Riccetti M, Gokey JJ, Aronow B, et al. . The elephant in the lung: integrating lineage-tracing, molecular markers, and single cell sequencing data to identify distinct fibroblast populations during lung development and regeneration. Matrix Biol 2020; 91–92: 51–74. doi:10.1016/j.matbio.2020.05.002 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
