Iron imbalance in neurodegeneration
- PMID: 38212377
- PMCID: PMC11176077
- DOI: 10.1038/s41380-023-02399-z
Iron imbalance in neurodegeneration
Abstract
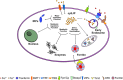
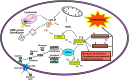
Iron is an essential element for the development and functionality of the brain, and anomalies in its distribution and concentration in brain tissue have been found to be associated with the most frequent neurodegenerative diseases. When magnetic resonance techniques allowed iron quantification in vivo, it was confirmed that the alteration of brain iron homeostasis is a common feature of many neurodegenerative diseases. However, whether iron is the main actor in the neurodegenerative process, or its alteration is a consequence of the degenerative process is still an open question. Because the different iron-related pathogenic mechanisms are specific for distinctive diseases, identifying the molecular mechanisms common to the various pathologies could represent a way to clarify this complex topic. Indeed, both iron overload and iron deficiency have profound consequences on cellular functioning, and both contribute to neuronal death processes in different manners, such as promoting oxidative damage, a loss of membrane integrity, a loss of proteostasis, and mitochondrial dysfunction. In this review, with the attempt to elucidate the consequences of iron dyshomeostasis for brain health, we summarize the main pathological molecular mechanisms that couple iron and neuronal death.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

