Lactic acid promotes nucleus pulposus cell senescence and corresponding intervertebral disc degeneration via interacting with Akt
- PMID: 38212432
- PMCID: PMC11071984
- DOI: 10.1007/s00018-023-05094-y
Lactic acid promotes nucleus pulposus cell senescence and corresponding intervertebral disc degeneration via interacting with Akt
Erratum in
-
Correction: Lactic acid promotes nucleus pulposus cell senescence and corresponding intervertebral disc degeneration via interacting with Akt.Cell Mol Life Sci. 2025 Aug 26;82(1):319. doi: 10.1007/s00018-025-05824-4. Cell Mol Life Sci. 2025. PMID: 40856852 Free PMC article. No abstract available.
Abstract
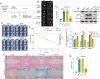
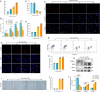
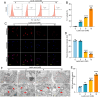
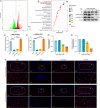
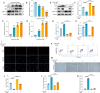
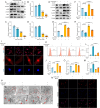
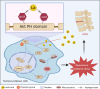
The accumulation of metabolites in the intervertebral disc is considered an important cause of intervertebral disc degeneration (IVDD). Lactic acid, which is a metabolite that is produced by cellular anaerobic glycolysis, has been proven to be closely associated with IVDD. However, little is known about the role of lactic acid in nucleus pulposus cells (NPCs) senescence and oxidative stress. The aim of this study was to investigate the effect of lactic acid on NPCs senescence and oxidative stress as well as the underlying mechanism. A puncture-induced disc degeneration (PIDD) model was established in rats. Metabolomics analysis revealed that lactic acid levels were significantly increased in degenerated intervertebral discs. Elimination of excessive lactic acid using a lactate oxidase (LOx)-overexpressing lentivirus alleviated the progression of IVDD. In vitro experiments showed that high concentrations of lactic acid could induce senescence and oxidative stress in NPCs. High-throughput RNA sequencing results and bioinformatic analysis demonstrated that the induction of NPCs senescence and oxidative stress by lactic acid may be related to the PI3K/Akt signaling pathway. Further study verified that high concentrations of lactic acid could induce NPCs senescence and oxidative stress by interacting with Akt and regulating its downstream Akt/p21/p27/cyclin D1 and Akt/Nrf2/HO-1 pathways. Utilizing molecular docking, site-directed mutation and microscale thermophoresis assays, we found that lactic acid could regulate Akt kinase activity by binding to the Lys39 and Leu52 residues in the PH domain of Akt. These results highlight the involvement of lactic acid in NPCs senescence and oxidative stress, and lactic acid may become a novel potential therapeutic target for the treatment of IVDD.
Keywords: Akt; Intervertebral disc degeneration; Lactic acid; Nucleus pulposus cell; Oxidative stress; Senescence.
© 2024. The Author(s), under exclusive licence to Springer Nature Switzerland AG.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Urban J, Smith S, Fairbank J (2004) Nutrition of the intervertebral disc, Spine, 29(23):2700-9, 10.1097/01.brs.0000146499.97948.52 - PubMed
-
- Holm S, Maroudas A, Urban J, Selstam G, Nachemson A (1981) Nutrition of the intervertebral disc: solute transport and metabolism, Connective tissue research, 8(2):101–19, 10.3109/03008208109152130 - PubMed
-
- Radek M et al (2016) Assessing the correlation between the degree of disc degeneration on the Pfirrmann scale and the metabolites identified in HR-MAS NMR spectroscopy, Magnetic resonance imaging, 34(4):376–80, 10.1016/j.mri.2015.12.005 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

