Accelarated immune ageing is associated with COVID-19 disease severity
- PMID: 38212801
- PMCID: PMC10782727
- DOI: 10.1186/s12979-023-00406-z
Accelarated immune ageing is associated with COVID-19 disease severity
Abstract
Background: The striking increase in COVID-19 severity in older adults provides a clear example of immunesenescence, the age-related remodelling of the immune system. To better characterise the association between convalescent immunesenescence and acute disease severity, we determined the immune phenotype of COVID-19 survivors and non-infected controls.
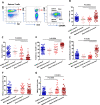
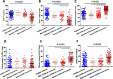
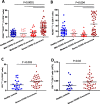
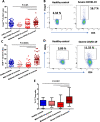
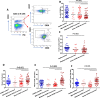
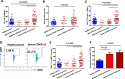
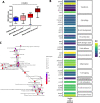
Results: We performed detailed immune phenotyping of peripheral blood mononuclear cells isolated from 103 COVID-19 survivors 3-5 months post recovery who were classified as having had severe (n = 56; age 53.12 ± 11.30 years), moderate (n = 32; age 52.28 ± 11.43 years) or mild (n = 15; age 49.67 ± 7.30 years) disease and compared with age and sex-matched healthy adults (n = 59; age 50.49 ± 10.68 years). We assessed a broad range of immune cell phenotypes to generate a composite score, IMM-AGE, to determine the degree of immune senescence. We found increased immunesenescence features in severe COVID-19 survivors compared to controls including: a reduced frequency and number of naïve CD4 and CD8 T cells (p < 0.0001); increased frequency of EMRA CD4 (p < 0.003) and CD8 T cells (p < 0.001); a higher frequency (p < 0.0001) and absolute numbers (p < 0.001) of CD28-ve CD57+ve senescent CD4 and CD8 T cells; higher frequency (p < 0.003) and absolute numbers (p < 0.02) of PD-1 expressing exhausted CD8 T cells; a two-fold increase in Th17 polarisation (p < 0.0001); higher frequency of memory B cells (p < 0.001) and increased frequency (p < 0.0001) and numbers (p < 0.001) of CD57+ve senescent NK cells. As a result, the IMM-AGE score was significantly higher in severe COVID-19 survivors than in controls (p < 0.001). Few differences were seen for those with moderate disease and none for mild disease. Regression analysis revealed the only pre-existing variable influencing the IMM-AGE score was South Asian ethnicity ([Formula: see text] = 0.174, p = 0.043), with a major influence being disease severity ([Formula: see text] = 0.188, p = 0.01).
Conclusions: Our analyses reveal a state of enhanced immune ageing in survivors of severe COVID-19 and suggest this could be related to SARS-Cov-2 infection. Our data support the rationale for trials of anti-immune ageing interventions for improving clinical outcomes in these patients with severe disease.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- The PHOSP-COVID Collaborative Group Clinical characteristics with inflammation profiling of Long-COVID and association with one-year recovery following hospitalisation in the UK: a prospective observational study. Lancet Resp Med. 2022;10(8):761–775. doi: 10.1016/s2213-2600(22)00127-8. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

