Fabry App: the value of a portable technology in recording day-to-day patient monitored information in patients with Fabry disease
- PMID: 38212814
- PMCID: PMC11057153
- DOI: 10.1186/s13023-023-02999-6
Fabry App: the value of a portable technology in recording day-to-day patient monitored information in patients with Fabry disease
Abstract
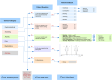
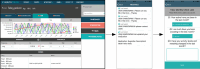
Background: Fabry disease is a rare inherited disorder resulting from deficient α-galactosidase A enzyme activity. Common disease manifestations are sweating abnormalities, neuropathic pain, gastrointestinal symptoms and fatigue. Challenges are faced by health care professionals in evaluating symptom burden in the current clinical setting, and the demand for alternative methods for monitoring disease-specific symptoms has seen an acceleration in recent years. Smartphone technologies offer the potential for continuity of care and surveillance. As a part of a quality improvement project, a disease specific app was developed in collaboration with a software company (Health Touch Ltd) and made available for patient use in May 2018. The Fabry mobile app records five categories: pain, gastrointestinal symptoms, sweating, activity levels, medications. Fabry disease patients with gastrointestinal and pain symptoms attending the Lysosomal Storage Disorders Unit of the Royal Free London NHS Foundation Trust were reviewed to assess eligibility and invited to download the app for recording their symptoms (activity, sweating, pain and gastrointestinal) and medications. Patient-generated data were transmitted to a secure website for clinicians to review.
Results: One-hundred and thirty-nine symptomatic Fabry disease patients who had a smartphone (iPhone or android) were invited to download the app. Sixty-seven patients (26 males and 41 females; median age, 49 years [range, 20-81]) downloaded and tracked the Fabry App at least once. The median frequency of use per patient was 6 (range, 1-629). Pain in the hands and abdominal pain were significantly higher (p = 0.009 and p = 0.007, respectively) in patients with classic phenotype compared with patients with non-classic phenotypes.
Conclusions: We demonstrated the feasibility and acceptability of a smartphone app to facilitate the remote assessment and monitoring of Fabry disease symptom burden on a daily/weekly basis, as an alternative to the current standard of care that requires patients to recall their symptoms during 6 to 12 monthly annual clinic visits. Patients who were more likely to use the app had greater disease burden. This innovation has the potential to assess disease progression, early therapeutic intervention, thereby decreasing the burden of morbidity and mortality among Fabry patients, and to record long-term effects of Fabry-specific therapies.
Keywords: Disease management; Disease monitoring; Fabry disease; Patient reported data; Smartphone applications.
© 2024. The Author(s).
Conflict of interest statement
SD: none related to this manuscript. SD is currently employed at Chiesi Farmaceutici S.p.A., unrelated to this project. MM none. DB is a patient and MH and AF are employees of Health Touch Ltd. GG none. UR: none related to this manuscript. UR has received research grants from Amicus, Takeda, Intrabio, Sanofi; Advisory board and lecture fees from Amicus, Takeda, Sanofi.
Figures

References
-
- Desnick RJ, Ioannou YA, Eng CM, et al. Alpha-galactosidase A deficiency: Fabry disease. In: Scriver CR, et al., editors. The metabolic and molecular bases of inherited disease. 8. New York: McGraw-Hill; 2001. pp. 3733–3774.
-
- Biegstraaten M, Arngrimsson R, Barbey F, Boks L, Cecchi F, Deegan PB, et al. Recommendations for initiation and cessation of enzyme replacement therapy in patients with Fabry disease: the European Fabry Working Group consensus document. Orphanet J Rare Dis. 2015;10:36. doi: 10.1186/s13023-015-0253-6. - DOI - PMC - PubMed
-
- Desnick RJ, Brady R, Barranger J, Collins AJ, Germain DP, Goldman M, et al. Fabry disease, an under-recognized multisystemic disorder: expert recommendations for diagnosis, management, and enzyme replacement therapy. Ann Intern Med. 2003;138(4):338–346. doi: 10.7326/0003-4819-138-4-200302180-00014. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

