Cognitive training and brain stimulation in patients with cognitive impairment: a randomized controlled trial
- PMID: 38212815
- PMCID: PMC10782634
- DOI: 10.1186/s13195-024-01381-3
Cognitive training and brain stimulation in patients with cognitive impairment: a randomized controlled trial
Abstract
Background: Repeated sessions of training and non-invasive brain stimulation have the potential to enhance cognition in patients with cognitive impairment. We hypothesized that combining cognitive training with anodal transcranial direct current stimulation (tDCS) will lead to performance improvement in the trained task and yield transfer to non-trained tasks.
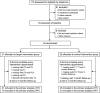
Methods: In our randomized, sham-controlled, double-blind study, 46 patients with cognitive impairment (60-80 years) were randomly assigned to one of two interventional groups. We administered a 9-session cognitive training (consisting of a letter updating and a Markov decision-making task) over 3 weeks with concurrent 1-mA anodal tDCS over the left dorsolateral prefrontal cortex (20 min in tDCS, 30 s in sham group). Primary outcome was trained task performance (letter updating task) immediately after training. Secondary outcomes included performance in tasks testing working memory (N-back task), decision-making (Wiener Matrices test) and verbal memory (verbal learning and memory test), and resting-state functional connectivity (FC). Tasks were administered at baseline, at post-assessment, and at 1- and 7-month follow-ups (FU). MRI was conducted at baseline and 7-month FU. Thirty-nine participants (85%) successfully completed the intervention. Data analyses are reported on the intention-to-treat (ITT) and the per-protocol (PP) sample.
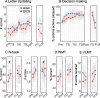
Results: For the primary outcome, no difference was observed in the ITT (β = 0.1, 95%-CI [- 1.2, 1.3, p = 0.93] or PP sample (β = - 0.2, 95%-CI [- 1.6, 1.2], p = 0.77). However, secondary analyses in the N-back working memory task showed that, only in the PP sample, the tDCS outperformed the sham group (PP: % correct, β = 5.0, 95%-CI [- 0.1, 10.2], p = 0.06, d-prime β = 0.2, 95%-CI [0.0, 0.4], p = 0.02; ITT: % correct, β = 3.0, 95%-CI [- 3.9, 9.9], p = 0.39, d-prime β = 0.1, 95%-CI [- 0.1, 0.3], p = 0.5). Frontoparietal network FC was increased from baseline to 7-month FU in the tDCS compared to the sham group (pFDR < 0.05). Exploratory analyses showed a correlation between individual memory improvements and higher electric field magnitudes induced by tDCS (ρtDCS = 0.59, p = 0.02). Adverse events did not differ between groups, questionnaires indicated successful blinding (incidence rate ratio, 1.1, 95%-CI [0.5, 2.2]).
Conclusions: In sum, cognitive training with concurrent brain stimulation, compared to cognitive training with sham stimulation, did not lead to superior performance enhancements in patients with cognitive impairment. However, we observed transferred working memory benefits in patients who underwent the full 3-week intervention. MRI data pointed toward a potential intervention-induced modulation of neural network dynamics. A link between individual performance gains and electric fields suggested dosage-dependent effects of brain stimulation. Together, our findings do not support the immediate benefit of the combined intervention on the trained function, but provide exploratory evidence for transfer effects on working memory in patients with cognitive impairment. Future research needs to explore whether individualized protocols for both training and stimulation parameters might further enhance treatment gains.
Trial registration: The study is registered on ClinicalTrials.gov (NCT04265378). Registered on 7 February 2020. Retrospectively registered.
Keywords: Electric field simulation; Mild cognitive impairment; Resting-state functional connectivity; Subjective cognitive decline; Transcranial direct current stimulation.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Cognitive training and brain stimulation in prodromal Alzheimer's disease (AD-Stim)-study protocol for a double-blind randomized controlled phase IIb (monocenter) trial.Alzheimers Res Ther. 2020 Nov 7;12(1):142. doi: 10.1186/s13195-020-00692-5. Alzheimers Res Ther. 2020. PMID: 33160420 Free PMC article.
-
Randomized trial of cognitive training and brain stimulation in non-demented older adults.Alzheimers Dement (N Y). 2022 Feb 23;8(1):e12262. doi: 10.1002/trc2.12262. eCollection 2022. Alzheimers Dement (N Y). 2022. PMID: 35229023 Free PMC article.
-
Neuromodulation through brain stimulation-assisted cognitive training in patients with post-chemotherapy subjective cognitive impairment (Neuromod-PCSCI) after breast cancer: study protocol for a double-blinded randomised controlled trial.BMJ Open. 2025 May 21;15(5):e096162. doi: 10.1136/bmjopen-2024-096162. BMJ Open. 2025. PMID: 40398955 Free PMC article.
-
The benefits of simultaneous tDCS and working memory training on transfer outcomes: A systematic review and meta-analysis.Brain Stimul. 2022 Nov-Dec;15(6):1541-1551. doi: 10.1016/j.brs.2022.11.008. Epub 2022 Nov 29. Brain Stimul. 2022. PMID: 36460294
-
Combined Cognitive Training and Transcranial Direct Current Stimulation in Neuropsychiatric Disorders: A Systematic Review and Meta-analysis.Biol Psychiatry Cogn Neurosci Neuroimaging. 2023 Feb;8(2):151-161. doi: 10.1016/j.bpsc.2022.09.014. Epub 2022 Oct 13. Biol Psychiatry Cogn Neurosci Neuroimaging. 2023. PMID: 36653210 Free PMC article.
Cited by
-
The Effectiveness of Computerized Cognitive Training in Patients With Poststroke Cognitive Impairment: Systematic Review and Meta-Analysis.J Med Internet Res. 2025 Jun 12;27:e73140. doi: 10.2196/73140. J Med Internet Res. 2025. PMID: 40503808 Free PMC article. Review.
-
The emerging field of non-invasive brain stimulation in Alzheimer's disease.Brain. 2024 Dec 3;147(12):4003-4016. doi: 10.1093/brain/awae292. Brain. 2024. PMID: 39562009 Free PMC article. Review.
-
Predicting the Beneficial Effects of Cognitive Stimulation and Transcranial Direct Current Stimulation in Amnestic Mild Cognitive Impairment with Clinical, Inflammation, and Human Microglia Exposed to Serum as Potential Markers: A Double-Blind Placebo-Controlled Randomized Clinical Trial.Int J Mol Sci. 2025 Feb 19;26(4):1754. doi: 10.3390/ijms26041754. Int J Mol Sci. 2025. PMID: 40004217 Free PMC article. Clinical Trial.
-
The Instantaneous Effect and Its Mechanism of Transcranial Direct Current Stimulation on Working Memory Based on Delta and Gamma Band Electroencephalography Characteristics.Brain Sci. 2025 May 27;15(6):579. doi: 10.3390/brainsci15060579. Brain Sci. 2025. PMID: 40563751 Free PMC article.
-
Prism adaptation combined with serious games for improving visual-constructive abilities in stroke patients: randomized clinical trial.Front Digit Health. 2025 Feb 21;7:1425410. doi: 10.3389/fdgth.2025.1425410. eCollection 2025. Front Digit Health. 2025. PMID: 40060034 Free PMC article.
References
-
- Smart CM, Karr JE, Areshenkoff CN, Rabin LA, Hudon C, Gates N, et al. Non-pharmacologic interventions for older adults with subjective cognitive decline: systematic review, meta-analysis, and preliminary recommendations. Neuropsychol Rev. 2017;27(3):245–257. doi: 10.1007/s11065-017-9342-8. - DOI - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

