Effect of Buteyko breathing technique on clinical and functional parameters in adult patients with asthma: a randomized, controlled study
- PMID: 38212823
- PMCID: PMC10782792
- DOI: 10.1186/s40001-023-01634-1
Effect of Buteyko breathing technique on clinical and functional parameters in adult patients with asthma: a randomized, controlled study
Abstract
Background: The established therapy of asthma might be supported by additional non-pharmaceutical measures, such as the Buteyko breathing technique (BBT); however, the available data are mixed. To clarify the effects of BBT in patients with asthma, we investigated whether it led to clinical improvements with correlation to functional parameters.
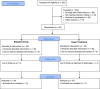
Methods: Using a randomized, controlled design, we studied two groups (n = 30 each) of patients with asthma under either BBT or usual therapy (UT) w/o BBT over a period of 3 months. The primary outcome comprised the voluntary control pause (CP) after 3 months, secondary outcomes an additional breathhold parameter, forced expiratory volume in 1 s (FEV1), capnovolumetry, exhaled nitric oxide (FeNO), Asthma Control Questionnaire (ACQ) and Nijmegen Questionnaire (NQ), and the use of medication (β2-agonists; inhaled corticosteroids, ICS).
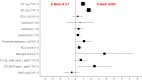
Results: CP showed significant time-by-group interaction [F(1,58.09) = 28.70, p < 0.001] as well as main effects for study group [F(1,58.27) = 5.91, p = 0.018] and time [F(1,58.36) = 17.67, p < 0.001]. ACQ and NQ scores were significantly (p < 0.05 each) improved with BBT. This was associated with reductions in the use of β2-agonists and ICS (p < 0.05 each) by about 20% each. None of these effects occurred in the UT group. While FEV1 and the slopes of the capnovolumetric expiratory phases 2 and 3 did not significantly change, the capnovolumetric threshold volume at tidal breathing increased (p < 0.05) with BBT by about 10 mL or 10%, compared to baseline, suggesting a larger volume of the central airways. No significant changes were seen for FeNO.
Conclusions: BBT was clinically effective, as indicated by the fact that the improvement in symptom scores and the small increase in bronchial volume occurred despite the significant reduction of respiratory pharmacotherapy. As the self-controlled Buteyko breathing therapy was well-accepted by the participants, it could be considered as supporting tool in asthma therapy being worth of wider attention in clinical practice. Trial registration Retrospectively registered on 10 March 2017 at ClinicalTrials.gov (NCT03098849).
Keywords: Asthma; Buteyko breathing technique; Capnovolumetry; Respiratory medication; Self-management; Symptoms.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests in relation to the work presented in this manuscript.
Figures
References
-
- GINA R. (2021). Global strategy for asthma management and prevention. https://ginasthma.org/wp-content/uploads/2021/04/GINA-2021-Main-Report_F.... - PubMed
-
- Witte JA, Braunstahl GJ, Blox WJB, van’t Westeinde SC, In’t Veen J, Kappen JH, et al. STOP: an open label crossover trial to study ICS withdrawal in patients with a combination of obesity and low-inflammatory asthma and evaluate its effect on asthma control and quality of life. BMC Pulm Med. 2022;22(1):53. doi: 10.1186/s12890-022-01843-0. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

