Sodium-glucose co-transporter-2 inhibitors in patients treated with immune checkpoint inhibitors
- PMID: 38212825
- PMCID: PMC10782769
- DOI: 10.1186/s40959-023-00199-6
Sodium-glucose co-transporter-2 inhibitors in patients treated with immune checkpoint inhibitors
Abstract
Background: Immune checkpoint inhibitors (ICIs) have revolutionized the prognosis of cancer. Diabetes mellitus (DM) has been shown to have a negative effect on patients treated with ICIs. Sodium-glucose cotransporter 2 inhibitors (SGLT2i) are effective antidiabetic therapies associated with reduced all-cause mortality and cardiovascular (CV) outcomes.
Objective: To evaluate the prognostic value of SGLT2i on all-cause mortality and cardiotoxicity among patients treated with ICIs.
Methods: We performed a retrospective analysis of patients diagnosed with cancer and type 2 DM (DM2) and treated with ICIs at our center. Patients were divided into two groups according to baseline treatment with or without SGLT2i. The primary endpoint was all-cause mortality and the secondary endpoint was MACE, including myocarditis, acute coronary syndrome, heart failure, and arrhythmia.
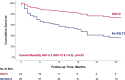
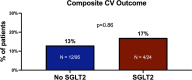
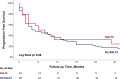
Results: The cohort included 119 patients, with 24 (20%) patients assigned to the SGLT2i group. Both groups exhibited a comparable prevalence of cardiac risk factors, although the SGLT2i group displayed a higher incidence of ischemic heart disease. Over a median follow-up of 28 months, 61 (51%) patients died, with a significantly lower all-cause mortality rate in the SGLT2i group (21% vs. 59%, p = 0.002). While there were no significant differences in MACE, we observed zero cases of myocarditis and atrial fibrillation in the SGLT2i, compared to 2 and 6 cases in the non-SGLT2i group.
Conclusions: SGLT2i therapy was associated with a lower all-cause mortality rate in patients diagnosed with cancer and DM2 and treated with ICIs. Further studies are needed to understand the mechanism and evaluate its benefit on cardiotoxicity.
Keywords: Cardio-oncology; Cardiotoxicity; Diabetes; ICIs; Immune checkpoint inhibitor; SGLT2.
© 2024. The Author(s).
Conflict of interest statement
All authors have nothing to disclose.
The study was approved by the local Helsinki committee (#TLV-0228–16).
Michal Laufer-Perl reports grants, consulting fees, and payment of lectures from of lectures from BI and AstraZeneca, and advisory board from BI. The other authors declare no conflict BI and AstraZeneca and advisory board form BI. Ofer Havakuk reports grants, consulting fees, payment of interest.
Figures
References
LinkOut - more resources
Full Text Sources