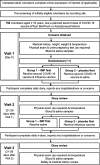
Delivering COVID-19 vaccine trials at speed: the implementation of a phase IV UK multi-centre randomised controlled trial to determine safety and immunogenicity of COVID-19 vaccines co-administered with seasonal influenza vaccines (ComFluCOV)
- PMID: 38212836
- PMCID: PMC10785514
- DOI: 10.1186/s13063-023-07862-4
Delivering COVID-19 vaccine trials at speed: the implementation of a phase IV UK multi-centre randomised controlled trial to determine safety and immunogenicity of COVID-19 vaccines co-administered with seasonal influenza vaccines (ComFluCOV)
Abstract
Background: In February 2021, the UK Department of Health and Social Care sought evidence on the safety and immunogenicity of COVID-19 and influenza vaccine co-administration to inform the 2021/2022 influenza vaccine policy. Co-administration could support vaccine uptake and reduce healthcare appointments. ComFluCOV was a randomised controlled trial designed to provide this evidence. This report outlines the methods used to deliver the trial in 6 months to answer an urgent public health question as part of the COVID-19 pandemic response.
Methods: ComFluCOV was commissioned by the Department of Health and Social Care and was managed by the Bristol Trials Centre, a UK-registered clinical trials unit. It was classed as an Urgent Public Health trial which facilitated fast-track regulatory approvals. Trial materials and databases were developed using in-house templates and those used in other COVID-19 vaccine trials. Participants were recruited by advertising, and via a trial website. Electronic trial systems enabled daily review of participant data. Weekly virtual meetings were held with stakeholders and trial sites.
Results: ComFluCOV was delivered within 6 months from inception to reporting, and trial milestones to inform the Department of Health and Social Care policy were met. Set-up was achieved within 1 month. Regulators provided expedited reviews, with feedback ahead of submission. Recruitment took place at 12 sites. Over 380 site staff were trained. Overall, 679 participants were recruited in two months. The final report to the Department of Health and Social Care was submitted in September 2021, following a preliminary safety report in May 2021. Trial results have been published.
Conclusion: The rapid delivery of ComFluCOV was resource intensive. It was made possible in part due to a unique set of circumstances created by the pandemic situation including measures put in place to support urgent public health research and public support for COVID-19 vaccine research. Elements of the trial could be adopted to increase efficiency in 'non-pandemic' situations including working with a clinical trials unit to enable immediate mobilisation of a team of experienced researchers, greater sharing of resources between clinical trials units, use of electronic trial systems and virtual meetings.
Trial registration: ISRCTN14391248, submitted on 17/03/2021. Registered on 30/03/2021.
Keywords: COVID-19 vaccine; Efficient delivery; Influenza vaccine; Randomised controlled trial (RCT); Urgent Public Health (UPH).
© 2024. The Author(s).
Conflict of interest statement
RL reports being the Principle and Chief Investigator for studies sponsored by commercial organisations including Moderna, Janssen, Astra-Zeneca, and Valneva. Consultation fees received for professional services funded by Sanofi and Astra-Zeneca. SB, LC and CAR reports grants from NIHR, during the conduct of the trial. AF reports grants from Pfizer during the conduct of the trial, and grants from Elizabeth Blackwell Institute, Sanofi Pasteur, VBI Vaccines, Pfizer, Janssen, GSK, MedImmune, Novavax, and Valneva outside the submitted work. All other authors, RTo, RTh, RH, JK, KJ, DH, MC and HCP, declare no competing interests.
Figures
Similar articles
-
Working under short timescales to deliver a national trial: a case study of the ComFluCOV trial from a statistician's perspective.Trials. 2024 Jan 23;25(1):79. doi: 10.1186/s13063-023-07879-9. Trials. 2024. PMID: 38263245 Free PMC article.
-
Comparison of assays used to detect antibody response in COVID-19 vaccine trials: Results from of a UK multi-Centre randomised controlled trial to determine the immunogenicity responses of COVID-19 vaccines administered concomitantly with seasonal influenza vaccines (ComFluCOV).Vaccine. 2024 Dec 2;42(26):126369. doi: 10.1016/j.vaccine.2024.126369. Epub 2024 Sep 23. Vaccine. 2024. PMID: 39316941 Clinical Trial.
-
A randomized, double-blind, placebo-controlled phase III clinical trial to evaluate the efficacy and safety of SARS-CoV-2 vaccine (inactivated, Vero cell): a structured summary of a study protocol for a randomised controlled trial.Trials. 2021 Apr 13;22(1):276. doi: 10.1186/s13063-021-05180-1. Trials. 2021. PMID: 33849629 Free PMC article.
-
Influenza virus vaccine live intranasal--MedImmune vaccines: CAIV-T, influenza vaccine live intranasal.Drugs R D. 2003;4(5):312-9. doi: 10.2165/00126839-200304050-00007. Drugs R D. 2003. PMID: 12952502 Review.
-
[Consideration on implementation of co-administration of Seasonal Influenza and COVID-19 vaccines during pandemic in China].Zhonghua Yu Fang Yi Xue Za Zhi. 2022 Feb 6;56(2):103-107. doi: 10.3760/cma.j.cn112150-20211203-01117. Zhonghua Yu Fang Yi Xue Za Zhi. 2022. PMID: 34954956 Review. Chinese.
Cited by
-
Progress in combination vaccines and the co-administration of influenza virus and SARS-CoV-2 vaccines.Front Immunol. 2025 Jun 25;16:1578733. doi: 10.3389/fimmu.2025.1578733. eCollection 2025. Front Immunol. 2025. PMID: 40636108 Free PMC article. Review.
-
Assessing vaccine coverage and delivery strategies for influenza and COVID-19 among Italian healthcare workers: A 2015-2023 case study.Hum Vaccin Immunother. 2025 Dec;21(1):2493027. doi: 10.1080/21645515.2025.2493027. Epub 2025 May 8. Hum Vaccin Immunother. 2025. PMID: 40338231 Free PMC article.
References
-
- Public Health England - COVID-19 vaccine surveillance report - Week 36. https://assets.publishing.service.gov.uk/government/uploads/system/uploa...; 2021.
-
- Feikin DR, Higdon MM, Abu-Raddad LJ, Andrews N, Araos R, Goldberg Y, et al. Duration of effectiveness of vaccines against SARS-CoV-2 infection and COVID-19 disease: results of a systematic review and meta-regression. Lancet. 2022;399(10328):924–44. doi: 10.1016/S0140-6736(22)00152-0. - DOI - PMC - PubMed
-
- National Institute of Health Research - Clinical Trials Guide https://www.nihr.ac.uk/documents/clinical-trials-guide/205952019.
-
- Raftery J, Young A, Stanton L, Milne R, Cook A, Turner D, Davidson P. Clinical trial metadata: defining and extracting metadata on the design, conduct, results and costs of 125 randomised clinical trials funded by the National Institute for Health Research Health Technology Assessment programme: Chapter 6Theme 3: performance and delivery of randomised controlled trials. Southampton (UK): NIHR Journals Library (Health Technology Assessment No 1911). 2015. - PMC - PubMed
-
- Lazarus R, Baos S, Cappel-Porter H, Carson-Stevens A, Clout M, Culliford L, et al. Safety and immunogenicity of concomitant administration of COVID-19 vaccines (ChAdOx1 or BNT162b2) with seasonal influenza vaccines in adults in the UK (ComFluCOV): a multicentre, randomised, controlled, phase 4 trial. Lancet. 2021;398(10318):2277–87. doi: 10.1016/S0140-6736(21)02329-1. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous