Sustain-release lipid-liquid crystal formulations of pexiganan against Helicobacter pylori infection: in vitro evaluation in C57BL/6 mice
- PMID: 38212864
- PMCID: PMC10785446
- DOI: 10.1186/s40360-024-00731-z
Sustain-release lipid-liquid crystal formulations of pexiganan against Helicobacter pylori infection: in vitro evaluation in C57BL/6 mice
Abstract
Introduction: The Gram-negative bacterium Helicobacter pylori, H. pylori, is associated with significant digestive disorders. However, the effectiveness of bacterial eradication is declining due to drug resistance. A potent anti-H. pylori activity is shown by the natural antimicrobial peptide pexiganan.
Objective: The current study aimed to evaluate the effectiveness of pexiganan and its lipid-liquid crystals (LLCs) in inducing Helicobacter pylori in mice.
Methods: In this experimental study, H. pylori infection was first induced in C57BL/6 mice. Secondly, the antibacterial efficacy of pexiganan and its LLCs formulations was investigated to eliminate H. pylori infection.
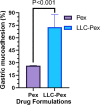
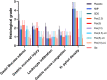
Results: The H. pylori infection could not be completely eradicated by pexiganan peptide alone. However, incorporating pexiganan within the LLC formulation resulted in an increased elimination of H. pylori. Under the H&E strain, the pexiganan-LLCs formulation revealed minimal mucosal alterations and a lower amount of inflammatory cell infiltration in the stomach compared to the placebo.
Conclusion: Clarithromycin was more effective than pexiganan at all tested concentrations. Furthermore, the pexiganan-loaded LLCs exhibited superior efficacy in curing H. pylori infection in a mouse model compared to pexiganan alone. This formulation can enhance H. pylori clearance while mitigating the adverse effects, typically associated with conventional drugs, leading to a viable alternative to current treatment options.
Keywords: C57BL/6; Cure rate: liquid crystal; Helicobacter pylori; Infection.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
The synthetic antimicrobial peptide pexiganan and its nanoparticles (PNPs) exhibit the anti-helicobacter pylori activity in vitro and in vivo.Molecules. 2015 Mar 2;20(3):3972-85. doi: 10.3390/molecules20033972. Molecules. 2015. PMID: 25738539 Free PMC article.
-
Effect of plaunotol in combination with clarithromycin or amoxicillin on Helicobacter pylori in vitro and in vivo.J Antimicrob Chemother. 2002 Jul;50(1):133-6. doi: 10.1093/jac/dkf094. J Antimicrob Chemother. 2002. PMID: 12096020
-
Antibacterial effect of Kampo herbal formulation Hochu-ekki-to (Bu-Zhong-Yi-Qi-Tang) on Helicobacter pylori infection in mice.Microbiol Immunol. 2002;46(7):475-82. doi: 10.1111/j.1348-0421.2002.tb02721.x. Microbiol Immunol. 2002. PMID: 12222933
-
Clarithromycin for treatment of Helicobacter pylori infections.Eur J Gastroenterol Hepatol. 1995 Aug;7 Suppl 1:S55-8. Eur J Gastroenterol Hepatol. 1995. PMID: 8574737 Review.
-
Clarithromycin and omeprazole as helicobacter pylori eradication therapy in patients with H. pylori-associated gastric disorders.Drugs. 1996 Jan;51(1):161-78. doi: 10.2165/00003495-199651010-00010. Drugs. 1996. PMID: 8741237 Review.
References
-
- Keikha M, Askari P, Ghazvini K, Karbalaei M. Levofloxacin-based therapy as an efficient alternative for eradicating Helicobacter pylori infection in Iran: a systematic review and meta-analysis. J Global Antimicrob Resist. 2021. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

