Low-dose fluconazole as a useful and safe prophylactic option in patients receiving allogeneic hematopoietic stem cell transplantation
- PMID: 38213090
- PMCID: PMC10905229
- DOI: 10.1002/cam4.6815
Low-dose fluconazole as a useful and safe prophylactic option in patients receiving allogeneic hematopoietic stem cell transplantation
Abstract
Background: Invasive fungal infections (IFIs) represent a potentially fatal complication in patients who undergo allogeneic hematopoietic stem cell transplantation (HSCT) if the initiation of therapy is delayed. Some guidelines recommend antifungal prophylaxis or preemptive therapy for these patients depending on the risk of IFIs following allogeneic HSCT. This retrospective study aimed to identify the group of patients who safely undergo allogeneic HSCT with low-dose fluconazole (FLCZ) prophylaxis (100 mg/day).
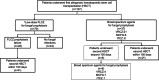
Methods: We retrospectively reviewed 107 patients who underwent their first allogeneic HSCT at Nagoya City University Hospital from January 1, 2010, to December 31, 2019. We analyzed the efficacy of low-dose FLCZ prophylaxis and investigated the relationship between major risk factors and antifungal prophylaxis failure (APF) within 100 days post-transplant.
Results: Of the 107 patients, 70 received low-dose FLCZ prophylaxis, showing a cumulative incidence of APF of 37.1% and a proven/probable IFI rate of 4.3%. There were no fungal infection-related deaths, including Aspergillus infections, in the FLCZ prophylaxis group. In a multivariable analysis, cord blood transplantation (CBT) (subdistribution hazard ratio (SHR), 3.55; 95% confidence interval (CI), 1.44-8.77; p = 0.006) and abnormal findings on lung CT before transplantation (SHR, 2.24; 95% CI, 1.02-4.92; p = 0.044) were independent risk factors for APF in the FLCZ prophylaxis group.
Conclusion: Low-dose FLCZ prophylaxis is a useful and safe option for patients receiving allogeneic HSCT, except in those undergoing CBT or having any fungal risk features including history of fungal infections, positive fungal markers, and abnormal findings on lung CT before transplantation.
Keywords: allogeneic hematopoietic stem cell transplantation; fluconazole; fungal infection; fungal prophylaxis.
© 2023 The Authors. Cancer Medicine published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

Similar articles
-
Management of Invasive Fungal Infections in Patients Undergoing Allogeneic Hematopoietic Stem Cell Transplantation: The Turin Experience.Front Cell Infect Microbiol. 2022 Jan 7;11:805514. doi: 10.3389/fcimb.2021.805514. eCollection 2021. Front Cell Infect Microbiol. 2022. PMID: 35071052 Free PMC article.
-
Randomized trial of micafungin versus fluconazole as prophylaxis against invasive fungal infections in hematopoietic stem cell transplant recipients.J Infect. 2016 Nov;73(5):496-505. doi: 10.1016/j.jinf.2016.06.011. Epub 2016 Jul 7. J Infect. 2016. PMID: 27394404 Clinical Trial.
-
Intravenous and oral itraconazole versus intravenous and oral fluconazole for long-term antifungal prophylaxis in allogeneic hematopoietic stem-cell transplant recipients. A multicenter, randomized trial.Ann Intern Med. 2003 May 6;138(9):705-13. doi: 10.7326/0003-4819-138-9-200305060-00006. Ann Intern Med. 2003. PMID: 12729424 Clinical Trial.
-
Primary antifungal prophylaxis in adult hematopoietic stem cell transplant recipients: current therapeutic concepts.Pharmacotherapy. 2009 Nov;29(11):1306-25. doi: 10.1592/phco.29.11.1306. Pharmacotherapy. 2009. PMID: 19857148 Review.
-
Antifungal prophylaxis in adult hematopoietic stem cell transplant recipients.Drugs Today (Barc). 2008 Jul;44(7):515-30. doi: 10.1358/dot.2008.44.7.1230943. Drugs Today (Barc). 2008. PMID: 18806902 Review.
Cited by
-
Antifungal Prophylaxis and Treatment of Breakthrough Invasive Fungal Diseases in High-Risk Hematology Patients: A Prospective Observational Multicenter Study.Indian J Hematol Blood Transfus. 2025 Jan;41(1):75-88. doi: 10.1007/s12288-024-01790-2. Epub 2024 May 20. Indian J Hematol Blood Transfus. 2025. PMID: 39917507
-
Efficacy of Low-Dose Fluconazole for Primary Prophylaxis of Invasive Candida Infections in Patients With Acute Leukemia: A Double-Blind Randomized Clinical Trial.Cancer Med. 2025 Apr;14(7):e70837. doi: 10.1002/cam4.70837. Cancer Med. 2025. PMID: 40152076 Free PMC article. Clinical Trial.
References
-
- Pagano L, Caira M, Candoni A, et al. The epidemiology of fungal infections in patients with hematologic malignancies: the SEIFEM‐2004 study. Haematologica. 2006;91:1068‐1075. - PubMed
-
- Pappas PG, Lionakis MS, Arendrup MC, Ostrosky‐Zeichner L, Kullberg BJ. Invasive candidiasis. Nat Rev Dis Primers. 2018;4:18026. - PubMed
-
- Thompson GR, Young JH. Aspergillus infections. N Engl J Med. 2021;385:1496‐1509. - PubMed
-
- Ziakas PD, Kourbeti IS, Mylonakis E. Systemic antifungal prophylaxis after hematopoietic stem cell transplantation: a meta‐analysis. Clin Ther. 2014;36:292‐306. - PubMed
-
- Slavin MA, Osborne B, Adams R, et al. Efficacy and safety of fluconazole prophylaxis for fungal infections after marrow transplantation ‐ a prospective, randomized, double‐blind study. J Infect Dis. 1995;171:1545‐1552. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

