Dynein directs prophase centrosome migration to control the stem cell division axis in the developing Caenorhabditis elegans epidermis
- PMID: 38213110
- PMCID: PMC11491518
- DOI: 10.1093/genetics/iyae005
Dynein directs prophase centrosome migration to control the stem cell division axis in the developing Caenorhabditis elegans epidermis
Abstract
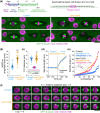
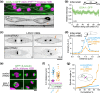
The microtubule motor dynein is critical for the assembly and positioning of mitotic spindles. In Caenorhabditis elegans, these dynein functions have been extensively studied in the early embryo but remain poorly explored in other developmental contexts. Here, we use a hypomorphic dynein mutant to investigate the motor's contribution to asymmetric stem cell-like divisions in the larval epidermis. Live imaging of seam cell divisions that precede formation of the seam syncytium shows that mutant cells properly assemble but frequently misorient their spindle. Misoriented divisions misplace daughter cells from the seam cell row, generate anucleate compartments due to aberrant cytokinesis, and disrupt asymmetric cell fate inheritance. Consequently, the seam becomes disorganized and populated with extra cells that have lost seam identity, leading to fatal epidermal rupture. We show that dynein orients the spindle through the cortical GOA-1Gα-LIN-5NuMA pathway by directing the migration of prophase centrosomes along the anterior-posterior axis. Spindle misorientation in the dynein mutant can be partially rescued by elongating cells, implying that dynein-dependent force generation and cell shape jointly promote correct asymmetric division of epithelial stem cells.
Keywords: Caenorhabditis elegans; LIN-5; dynein; epidermis; spindle orientation; stem cells.
© The Author(s) 2024. Published by Oxford University Press on behalf of The Genetics Society of America. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Conflict of interest statement
Conflicts of interest The authors declare no conflicts of interest.
Figures






Similar articles
-
Visualization of dynein-dependent microtubule gliding at the cell cortex: implications for spindle positioning.J Cell Biol. 2011 Aug 8;194(3):377-86. doi: 10.1083/jcb.201103128. J Cell Biol. 2011. PMID: 21825072 Free PMC article.
-
The polarity-induced force imbalance in Caenorhabditis elegans embryos is caused by asymmetric binding rates of dynein to the cortex.Mol Biol Cell. 2018 Dec 15;29(26):3093-3104. doi: 10.1091/mbc.E17-11-0653. Epub 2018 Oct 17. Mol Biol Cell. 2018. PMID: 30332325 Free PMC article.
-
Dynein localization and pronuclear movement in the C. elegans zygote.Cytoskeleton (Hoboken). 2022 Dec;79(12):133-143. doi: 10.1002/cm.21733. Epub 2022 Oct 28. Cytoskeleton (Hoboken). 2022. PMID: 36214774 Free PMC article.
-
Mechanisms of spindle positioning: cortical force generators in the limelight.Curr Opin Cell Biol. 2013 Dec;25(6):741-8. doi: 10.1016/j.ceb.2013.07.008. Epub 2013 Aug 16. Curr Opin Cell Biol. 2013. PMID: 23958212 Review.
-
Spindle positioning during the asymmetric first cell division of Caenorhabditis elegans embryos.Novartis Found Symp. 2001;237:164-75; discussion 176-81. doi: 10.1002/0470846666.ch13. Novartis Found Symp. 2001. PMID: 11444042 Review.
References
-
- Barbosa DJ, Duro J, Prevo B, Cheerambathur DK, Carvalho AX, Gassmann R. 2017. Dynactin binding to tyrosinated microtubules promotes centrosome centration in C. elegans by enhancing dynein-mediated organelle transport. Plos Genet. 13(7):e1006941. doi:10.1371/journal.pgen.1006941. - DOI - PMC - PubMed
-
- Celestino R, Henen MA, Gama JB, Carvalho C, McCabe M, Barbosa DJ, Born A, Nichols PJ, Carvalho AX, Gassmann R, et al. 2019. A transient helix in the disordered region of dynein light intermediate chain links the motor to structurally diverse adaptors for cargo transport. Plos Biol. 17(1):e3000100. doi:10.1371/journal.pbio.3000100. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

