Therapeutic significance of tumor microenvironment in cholangiocarcinoma: focus on tumor-infiltrating T lymphocytes
- PMID: 38213535
- PMCID: PMC10776604
- DOI: 10.37349/etat.2023.00199
Therapeutic significance of tumor microenvironment in cholangiocarcinoma: focus on tumor-infiltrating T lymphocytes
Abstract
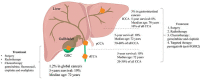
Cholangiocarcinoma (CCA) is a highly aggressive type of adenocarcinoma distinguished by its invasiveness. Depending on specific anatomical positioning within the biliary tree, CCA can be categorized into intrahepatic CCA (ICCA), perihilar CCA (pCCA) and distal CCA (dCCA). In recent years, there has been a significant increase in the global prevalence of CCA. Unfortunately, many CCA patients are diagnosed at an advanced stage, which makes surgical resection impossible. Although systemic chemotherapy is frequently used as the primary treatment for advanced or recurrent CCA, its effectiveness is relatively low. Therefore, immunotherapy has emerged as a promising avenue for advancing cancer treatment research. CCA exhibits a complex immune environment within the stromal tumor microenvironment (TME), comprising a multifaceted immune landscape and a tumor-reactive stroma. A deeper understanding of this complex TME is indispensable for identifying potential therapeutic targets. Thus, targeting tumor immune microenvironment holds promise as an effective therapeutic strategy.
Keywords: Tumor microenvironment; cholangiocarcinoma; immunotherapy; molecular pathogenesis; tumor-infiltrating T lymphocytes.
© The Author(s) 2023.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures

Similar articles
-
The immune milieu of cholangiocarcinoma: From molecular pathogenesis to precision medicine.J Autoimmun. 2019 Jun;100:17-26. doi: 10.1016/j.jaut.2019.03.007. Epub 2019 Mar 9. J Autoimmun. 2019. PMID: 30862450 Review.
-
New and Emerging Systemic Therapeutic Options for Advanced Cholangiocarcinoma.Cells. 2020 Mar 11;9(3):688. doi: 10.3390/cells9030688. Cells. 2020. PMID: 32168869 Free PMC article. Review.
-
The role of tumor-infiltrating lymphocytes in cholangiocarcinoma.J Exp Clin Cancer Res. 2022 Apr 7;41(1):127. doi: 10.1186/s13046-022-02340-2. J Exp Clin Cancer Res. 2022. PMID: 35392957 Free PMC article. Review.
-
Role of Cancer Stem Cells in Cholangiocarcinoma and Therapeutic Implications.Int J Mol Sci. 2019 Aug 25;20(17):4154. doi: 10.3390/ijms20174154. Int J Mol Sci. 2019. PMID: 31450710 Free PMC article. Review.
-
Clinical treatment of cholangiocarcinoma: an updated comprehensive review.Ann Hepatol. 2022 Sep-Oct;27(5):100737. doi: 10.1016/j.aohep.2022.100737. Epub 2022 Jul 7. Ann Hepatol. 2022. PMID: 35809836 Review.
Cited by
-
Advancing Cholangiocarcinoma Care: Insights and Innovations in T Cell Therapy.Cancers (Basel). 2024 Sep 23;16(18):3232. doi: 10.3390/cancers16183232. Cancers (Basel). 2024. PMID: 39335203 Free PMC article. Review.
References
-
- Banales JM, Cardinale V, Carpino G, Marzioni M, Andersen JB, Invernizzi P, et al. Cholangiocarcinoma: current knowledge and future perspectives consensus statement from the European Network for the Study of Cholangiocarcinoma (ENS-CCA). Nat Rev Gastroenterol Hepatol. 2016;13:261–80. doi: 10.1038/nrgastro.2016.51. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
