Prothymosin α accelerates dengue virus-induced thrombocytopenia
- PMID: 38213625
- PMCID: PMC10783621
- DOI: 10.1016/j.isci.2023.108422
Prothymosin α accelerates dengue virus-induced thrombocytopenia
Abstract
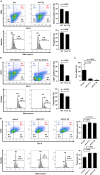
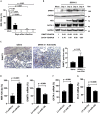
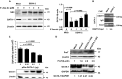
Thrombocytopenia is the hallmark finding in dengue virus (DENV) infection. Prothymosin α (ProT) has both intracellular and extracellular functions involved in cell cycle progression, cell differentiation, gene regulation, oxidative stress response, and immunomodulation. In this study, we found that ProT levels were elevated in dengue patient sera as well as DENV-infected megakaryoblasts and their culture supernatants. ProT transgenic mice had reduced platelet counts with prolonged bleeding times. Upon treatment with DENV plus anti-CD41 antibody, they exhibited severe skin hemorrhage. Furthermore, overexpression of ProT suppressed megakaryocyte differentiation. Infection with DENV inhibited miR-126 expression, upregulated DNA (cytosine-5)-methyltransferase 1 (DNMT1), downregulated GATA-1, and increased ProT expression. Upregulation of ProT led to Nrf2 activation and reduced reactive oxygen species production, thereby suppressing megakaryopoiesis. We report the pathophysiological role of ProT in DENV infection and propose an involvement of the miR-126-DNMT1-GATA-1-ProT-Nrf2 signaling axis in DENV-induced thrombocytopenia.
Keywords: Cell biology; Virology.
© 2023 The Authors.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures





Similar articles
-
Dengue Virus Targets Nrf2 for NS2B3-Mediated Degradation Leading to Enhanced Oxidative Stress and Viral Replication.J Virol. 2020 Nov 23;94(24):e01551-20. doi: 10.1128/JVI.01551-20. Print 2020 Nov 23. J Virol. 2020. PMID: 32999020 Free PMC article.
-
Dengue virus infection impedes megakaryopoiesis in MEG-01 cells where the virus envelope protein interacts with the transcription factor TAL-1.Sci Rep. 2020 Nov 11;10(1):19587. doi: 10.1038/s41598-020-76350-5. Sci Rep. 2020. PMID: 33177556 Free PMC article.
-
Dengue virus nonstructural protein 1 activates platelets via Toll-like receptor 4, leading to thrombocytopenia and hemorrhage.PLoS Pathog. 2019 Apr 22;15(4):e1007625. doi: 10.1371/journal.ppat.1007625. eCollection 2019 Apr. PLoS Pathog. 2019. PMID: 31009511 Free PMC article.
-
Platelets in dengue infection: more than a numbers game.Platelets. 2022 Feb 17;33(2):176-183. doi: 10.1080/09537104.2021.1921722. Epub 2021 May 24. Platelets. 2022. PMID: 34027810 Review.
-
Dengue Virus and Platelets: From the Biology to the Clinic.Viral Immunol. 2022 Jun;35(5):349-358. doi: 10.1089/vim.2021.0135. Epub 2022 Apr 28. Viral Immunol. 2022. PMID: 35483090 Review.
Cited by
-
Direct fibroblast reprogramming: an emerging strategy for treating organic fibrosis.J Transl Med. 2025 Feb 27;23(1):240. doi: 10.1186/s12967-024-06060-3. J Transl Med. 2025. PMID: 40016790 Free PMC article. Review.
-
Recent advances and current challenges of new approach methodologies in developmental and adult neurotoxicity testing.Arch Toxicol. 2024 May;98(5):1271-1295. doi: 10.1007/s00204-024-03703-8. Epub 2024 Mar 13. Arch Toxicol. 2024. PMID: 38480536 Free PMC article. Review.
References
-
- Wang S., He R., Patarapotikul J., Innis B.L., Anderson R. Antibody-enhanced binding of dengue-2 virus to human platelets. Virology. 1995;213:254–257. - PubMed
-
- Saito M., Oishi K., Inoue S., Dimaano E.M., Alera M.T.P., Robles A.M.P., Estrella B.D., Jr., Kumatori A., Moji K., Alonzo M.T., et al. Association of increased platelet-associated immunoglobulins with thrombocytopenia and the severity of disease in secondary dengue virus infections. Clin. Exp. Immunol. 2004;138:299–303. - PMC - PubMed
-
- Nelson E.R., Bierman H.R., Chulajata R. Hematologic findings in the 1960 hemorrhagic fever epidemic (dengue) in Thailand. Am. J. Trop. Med. Hyg. 1964;13:642–649. - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

