Pre- and postsynaptic nanostructures increase in size and complexity after induction of long-term potentiation
- PMID: 38213627
- PMCID: PMC10783556
- DOI: 10.1016/j.isci.2023.108679
Pre- and postsynaptic nanostructures increase in size and complexity after induction of long-term potentiation
Abstract
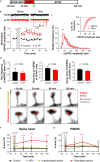
Synapses, specialized contact sites between neurons, are the fundamental elements of neuronal information transfer. Synaptic plasticity involves changes in synaptic morphology and the number of neurotransmitter receptors, and is thought to underlie learning and memory. However, it is not clear how these structural and functional changes are connected. We utilized time-lapse super-resolution STED microscopy of organotypic hippocampal brain slices and cultured neurons to visualize structural changes of the synaptic nano-organization of the postsynaptic scaffolding protein PSD95, the presynaptic scaffolding protein Bassoon, and the GluA2 subunit of AMPA receptors by chemically induced long-term potentiation (cLTP) at the level of single synapses. We found that the nano-organization of all three proteins increased in complexity and size after cLTP induction. The increase was largely synchronous, peaking at ∼60 min after stimulation. Therefore, both the size and complexity of individual pre- and post-synaptic nanostructures serve as substrates for tuning and determining synaptic strength.
Keywords: Neuroscience; Sensory neuroscience.
© 2023 The Authors.
Conflict of interest statement
The authors declare no competing interests.
Figures





Similar articles
-
Activity-Dependent Remodeling of Synaptic Protein Organization Revealed by High Throughput Analysis of STED Nanoscopy Images.Front Neural Circuits. 2020 Oct 15;14:57. doi: 10.3389/fncir.2020.00057. eCollection 2020. Front Neural Circuits. 2020. PMID: 33177994 Free PMC article.
-
Differential Scaling of Synaptic Molecules within Functional Zones of an Excitatory Synapse during Homeostatic Plasticity.eNeuro. 2020 Apr 23;7(2):ENEURO.0407-19.2020. doi: 10.1523/ENEURO.0407-19.2020. Print 2020 Mar/Apr. eNeuro. 2020. PMID: 32184300 Free PMC article.
-
N-terminal SAP97 isoforms differentially regulate synaptic structure and postsynaptic surface pools of AMPA receptors.Hippocampus. 2017 Jun;27(6):668-682. doi: 10.1002/hipo.22723. Epub 2017 Mar 20. Hippocampus. 2017. PMID: 28244171
-
Linking Nanoscale Dynamics of AMPA Receptor Organization to Plasticity of Excitatory Synapses and Learning.J Neurosci. 2018 Oct 31;38(44):9318-9329. doi: 10.1523/JNEUROSCI.2119-18.2018. J Neurosci. 2018. PMID: 30381423 Free PMC article. Review.
-
Cell adhesion and homeostatic synaptic plasticity.Neuropharmacology. 2014 Mar;78:23-30. doi: 10.1016/j.neuropharm.2013.03.015. Epub 2013 Mar 28. Neuropharmacology. 2014. PMID: 23542441 Review.
Cited by
-
Loss of postsynaptic NMDARs drives nanoscale reorganization of Munc13-1 and PSD-95.bioRxiv [Preprint]. 2024 Jan 12:2024.01.12.574705. doi: 10.1101/2024.01.12.574705. bioRxiv. 2024. PMID: 38260705 Free PMC article. Preprint.
-
Mitigation of synaptic and memory impairments via F-actin stabilization in Alzheimer's disease.Alzheimers Res Ther. 2024 Sep 7;16(1):200. doi: 10.1186/s13195-024-01558-w. Alzheimers Res Ther. 2024. PMID: 39244567 Free PMC article.
-
Astrocyte coverage of excitatory synapses correlates to measures of synapse structure and function in ferret primary visual cortex.Glia. 2024 Oct;72(10):1785-1800. doi: 10.1002/glia.24582. Epub 2024 Jun 10. Glia. 2024. PMID: 38856149
-
Dynamic extracellular interactions with AMPA receptors.bioRxiv [Preprint]. 2025 Jul 14:2025.07.11.664166. doi: 10.1101/2025.07.11.664166. bioRxiv. 2025. PMID: 40791527 Free PMC article. Preprint.
-
Learning-induced remodelling of inhibitory synapses in the motor cortex.Open Biol. 2024 Nov;14(11):240109. doi: 10.1098/rsob.240109. Epub 2024 Nov 13. Open Biol. 2024. PMID: 39532150 Free PMC article.
References
-
- Calverley R.K., Jones D.G. A serial-section study of perforated synapses in rat neocortex. Cell Tissue Res. 1987;247:565–572. - PubMed
LinkOut - more resources
Full Text Sources

