Association of latent factors of neuroinflammation with Alzheimer's disease pathology and longitudinal cognitive decline
- PMID: 38213951
- PMCID: PMC10781650
- DOI: 10.1002/dad2.12510
Association of latent factors of neuroinflammation with Alzheimer's disease pathology and longitudinal cognitive decline
Abstract
Introduction: We investigated the association of inflammatory mechanisms with markers of Alzheimer's disease (AD) pathology and rates of cognitive decline in the AD spectrum.
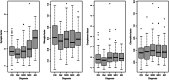
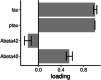
Methods: We studied 296 cases from the Deutsches Zentrum für Neurodegenerative Erkrankungen Longitudinal Cognitive Impairment and Dementia Study (DELCODE) cohort, and an extension cohort of 276 cases of the Alzheimer's Disease Neuroimaging Initiative study. Using Bayesian confirmatory factor analysis, we constructed latent factors for synaptic integrity, microglia, cerebrovascular endothelial function, cytokine/chemokine, and complement components of the inflammatory response using a set of inflammatory markers in cerebrospinal fluid.
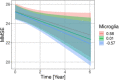
Results: We found strong evidence for an association of synaptic integrity, microglia response, and cerebrovascular endothelial function with a latent factor of AD pathology and with rates of cognitive decline. We found evidence against an association of complement and cytokine/chemokine factors with AD pathology and rates of cognitive decline.
Discussion: Latent factors provided access to directly unobservable components of the neuroinflammatory response and their association with AD pathology and cognitive decline.
Keywords: amyloid; chemokine factors; complement; endothelial function; microglia; structural equation models; tau.
© 2024 The Authors. Alzheimer's & Dementia: Diagnosis, Assessment & Disease Monitoring published by Wiley Periodicals LLC on behalf of Alzheimer's Association.
Conflict of interest statement
S.J.T. participated in scientific advisory boards of Roche Pharma AG, Biogen, Grifols, and MSD, and received lecture fees from Roche and MSD. M.T.H. serves as a scientific board member of IFM Therapeutics, Novo Nordisk, and Alector and has received lecture honoraria from NovoNordisk. The other authors state no competing interests. Author disclosures are available in the supporting information.
Figures





References
-
- Heneka MT, O'Banion MK. Inflammatory processes in Alzheimer's disease. J Neuroimmunol. 2007;184:69‐91. - PubMed
-
- Calsolaro V, Edison P. Neuroinflammation in Alzheimer's disease: current evidence and future directions. Alzheimers Dement. 2016;12:719‐732. - PubMed
-
- Cai Z, Hussain MD, Yan LJ. Microglia, neuroinflammation, and beta‐amyloid protein in Alzheimer's disease. Int J Neurosci. 2014;124:307‐321. - PubMed
-
- Heneka MT, O'Banion MK, Terwel D, Kummer MP. Neuroinflammatory processes in Alzheimer's disease. J Neural Transm (Vienna). 2010;117:919‐947. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
