Selective fluorescence turn-on detection of combination cisplatin-etoposide chemotherapy based on N-CDs/GSH-CuNCs nanoprobe
- PMID: 38213979
- PMCID: PMC10783161
- DOI: 10.1039/d3ra07844b
Selective fluorescence turn-on detection of combination cisplatin-etoposide chemotherapy based on N-CDs/GSH-CuNCs nanoprobe
Abstract
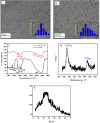
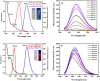
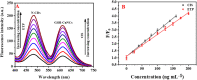
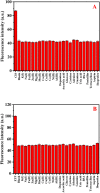
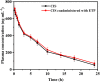
Cisplatin (CIS) and etoposide (ETP) combination therapy is highly effective for treating various cancers. However, the potential for pharmacokinetic interactions between these drugs necessitates selective sensing methods to quantitate both CIS and ETP levels in patient's plasma. This work develops a dual fluorescence probe strategy using glutathione-capped copper nanoclusters (GSH-CuNCs) and nitrogen-doped carbon dots (N-CDs) for the simultaneous analysis of CIS and ETP. The fluorescence signal of GSH-CuNCs at 615 nm increased linearly with CIS concentration while the N-CD emission at 480 nm remained unaffected. Conversely, the N-CD fluorescence was selectively enhanced by ETP with no interference with the CuNC fluorescence. Extensive materials characterization including UV-vis, fluorescence spectroscopy, XRD, and TEM confirmed the synthesis of the nanoprobes. The sensor showed high sensitivity with limits of detection of 6.95 ng mL-1 for CIS and 7.63 ng mL-1 for ETP along with excellent selectivity against potential interferences in rabbit plasma. Method feasibility was demonstrated with application to real rabbit plasma samples. The method was further applied to estimate the pharmacokinetic parameters of CIS before and after ETP coadministration. The dual nanoprobe sensing strategy enables rapid and selective quantitation of CIS and ETP levels to facilitate therapeutic drug monitoring and optimization of combination chemotherapy regimens.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
Similar articles
-
A novel ratiometric fluorescence nanoprobe for sensitive determination of uric acid based on CD@ZIF-CuNC nanocomposites.Mikrochim Acta. 2021 Jul 16;188(8):259. doi: 10.1007/s00604-021-04914-x. Mikrochim Acta. 2021. PMID: 34268632
-
Ratiometric fluorescence probe of Cu2+ and biothiols by using carbon dots and copper nanoclusters.RSC Adv. 2021 Oct 15;11(53):33662-33674. doi: 10.1039/d1ra05854a. eCollection 2021 Oct 8. RSC Adv. 2021. PMID: 35497542 Free PMC article.
-
A dual-emitting fluoroprobe fabricated by aloe leaf-based N-doped carbon quantum dots and copper nanoclusters for nitenpyram detection in waters by virtue of inner filter effect and static quenching principles.Anal Chim Acta. 2024 Feb 8;1289:342182. doi: 10.1016/j.aca.2023.342182. Epub 2024 Jan 3. Anal Chim Acta. 2024. PMID: 38245198
-
Self-assembly of DNA-templated copper nanoclusters and carbon dots for ratiometric fluorometric and visual determination of arginine and acetaminophen with a logic-gate operation.Mikrochim Acta. 2020 Feb 4;187(3):154. doi: 10.1007/s00604-020-4146-6. Mikrochim Acta. 2020. PMID: 32020297
-
A Dual Mode Foolproof Sensor Based on Glutathione Stabilized Copper Nanoclusters for Mercuric Ions and its Application in Fluorescence Sensing of Ascorbic acid.J Fluoresc. 2025 May 21. doi: 10.1007/s10895-025-04342-7. Online ahead of print. J Fluoresc. 2025. PMID: 40397337
Cited by
-
Utilizing a graphene quantum dot/hydrogel nanocomposite for determination of cisplatin in urine samples.RSC Adv. 2024 Aug 13;14(35):25329-25336. doi: 10.1039/d4ra04294h. eCollection 2024 Aug 12. RSC Adv. 2024. PMID: 39139239 Free PMC article.
-
A novel urease-assisted ratiometric fluorescence sensing platform based on pH-modulated copper-quenched near-infrared carbon dots and methyl red-quenched red carbon dots for selective urea monitoring.Mikrochim Acta. 2024 Aug 4;191(8):505. doi: 10.1007/s00604-024-06573-0. Mikrochim Acta. 2024. PMID: 39097544
-
Ratiometric Sensing of Azithromycin and Sulfide Using Dual Emissive Carbon Dots: A Turn On-Off-On Approach.J Fluoresc. 2025 May;35(5):2979-2991. doi: 10.1007/s10895-024-03737-2. Epub 2024 May 1. J Fluoresc. 2025. PMID: 38691279
References
-
- Gershenson D. M., Lentz G. M., Valea F. A. and Lobo R. A., Comprehensive Gynecology, Elsevier, St. Louis (MO), 8th edn, 2022, pp. 618–636
-
- Agrawal K., in xPharm: The Comprehensive Pharmacology Reference, ed. S. J. Enna and D. B. Bylund, Elsevier, New York, 2007, pp. 1–5
LinkOut - more resources
Full Text Sources

