Comparative Analysis of the Donor Properties of Isomeric Pyrrolyl Phosphine Ligands
- PMID: 38213984
- PMCID: PMC10777409
- DOI: 10.1021/acs.organomet.3c00467
Comparative Analysis of the Donor Properties of Isomeric Pyrrolyl Phosphine Ligands
Abstract
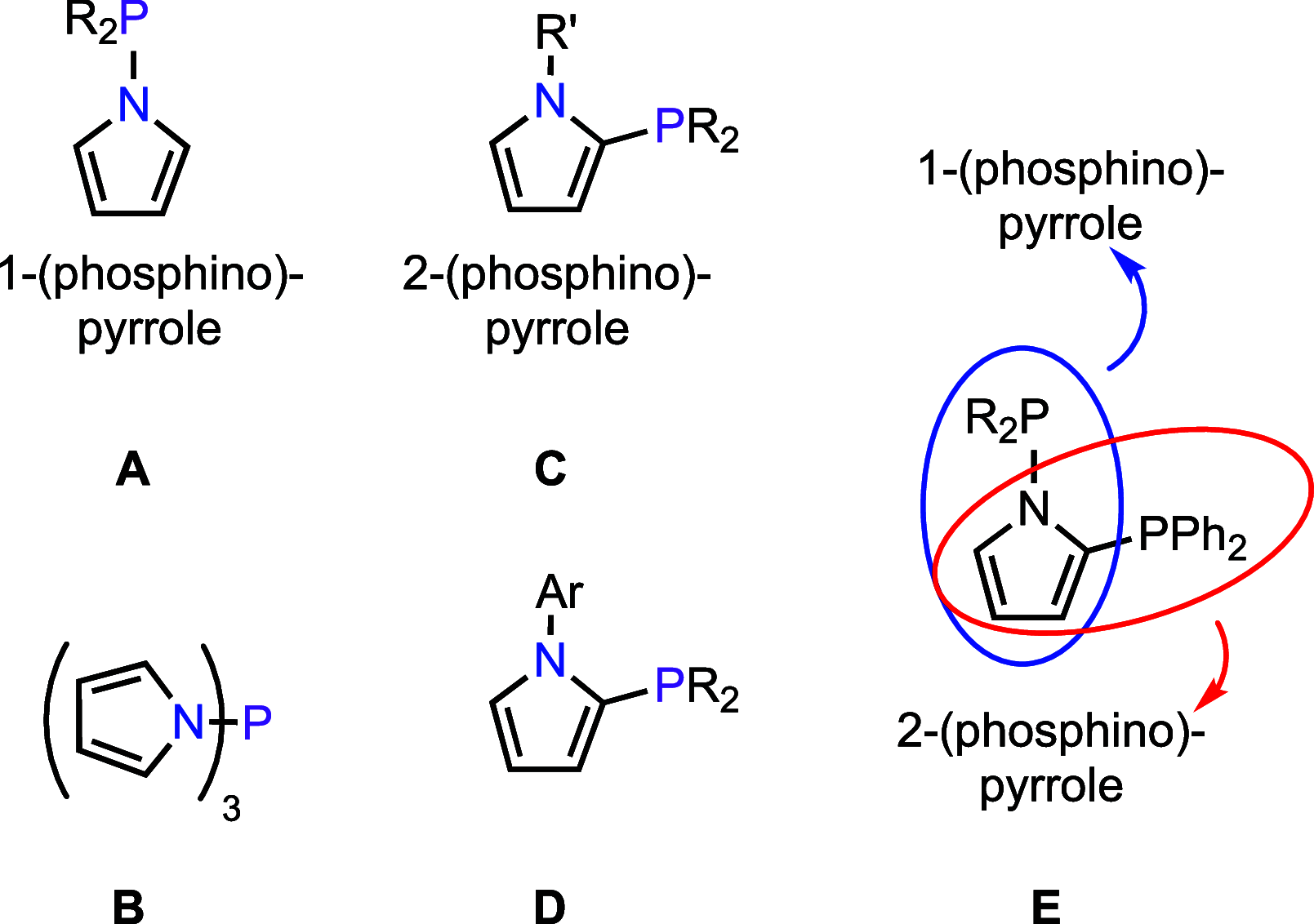
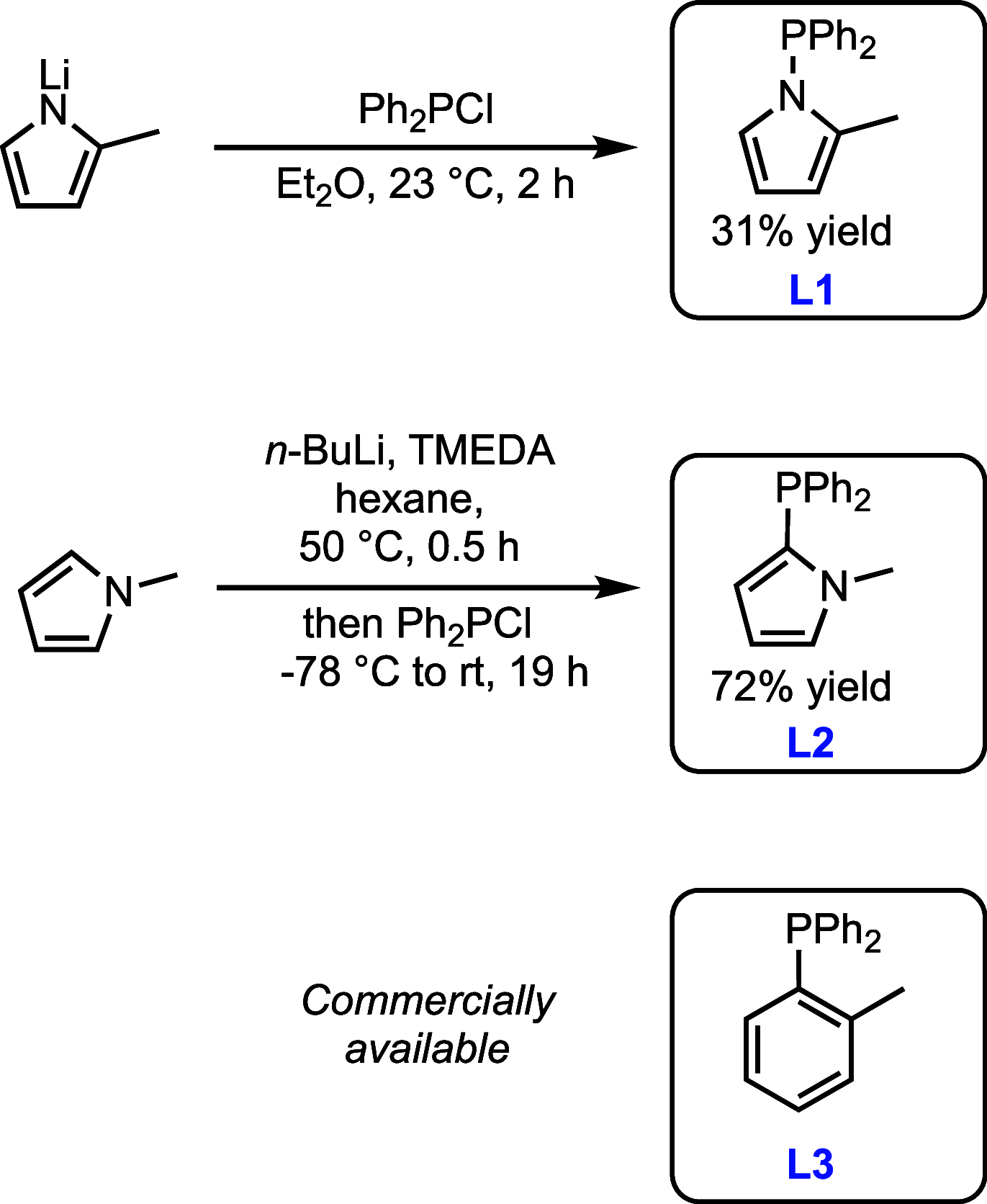
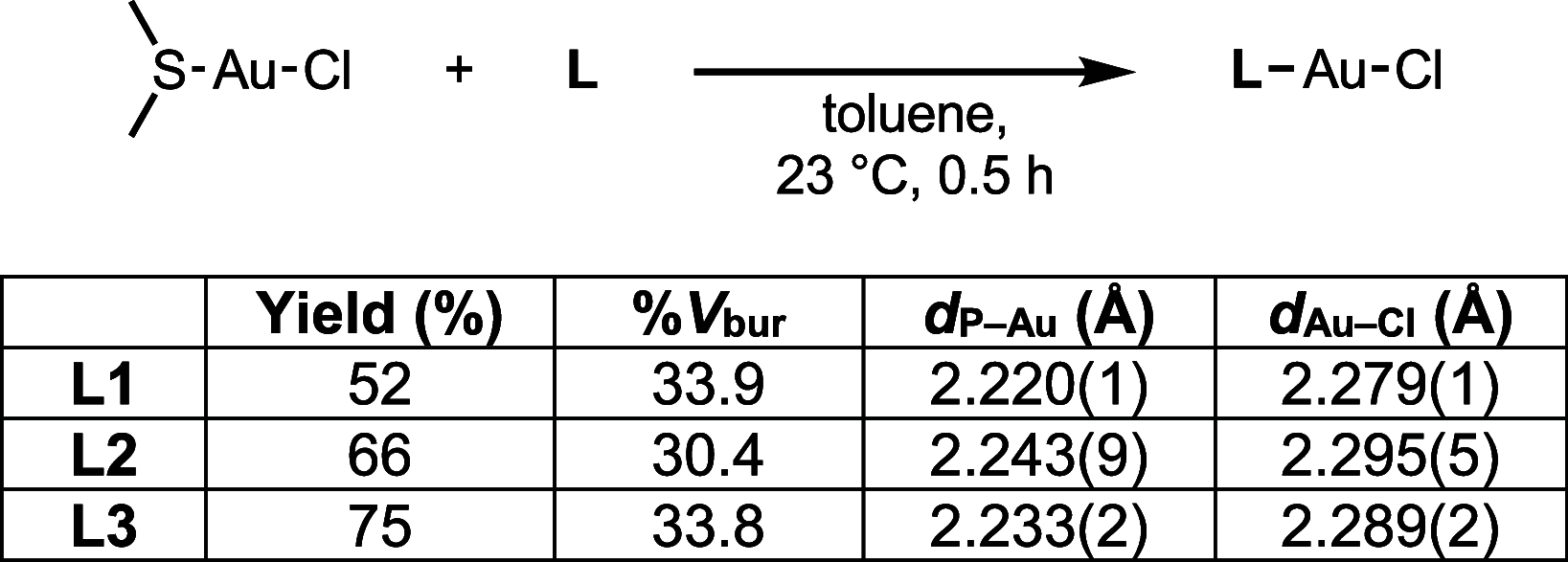
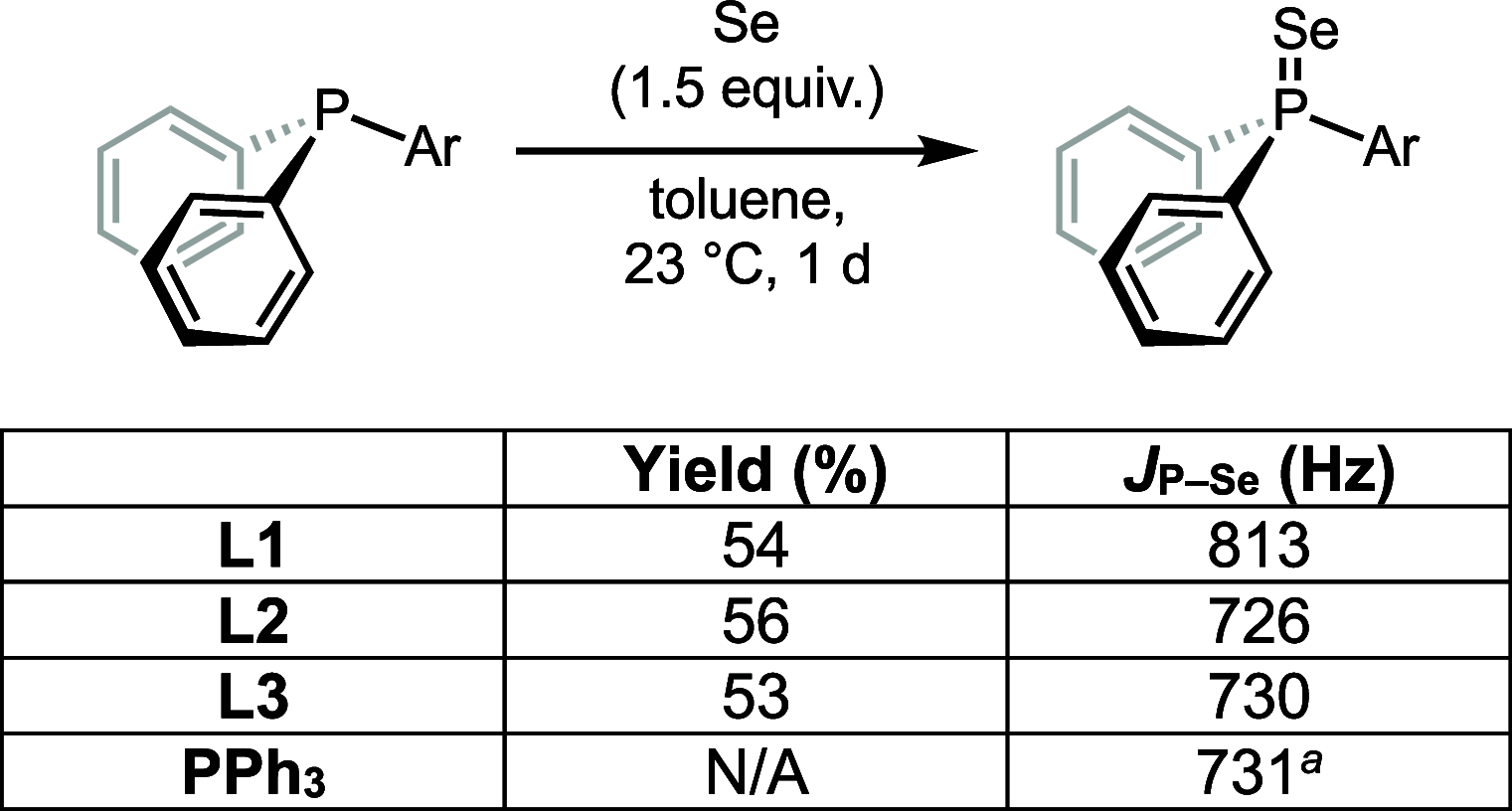
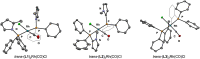
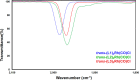
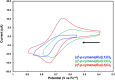
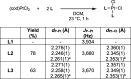
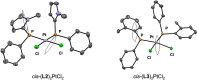
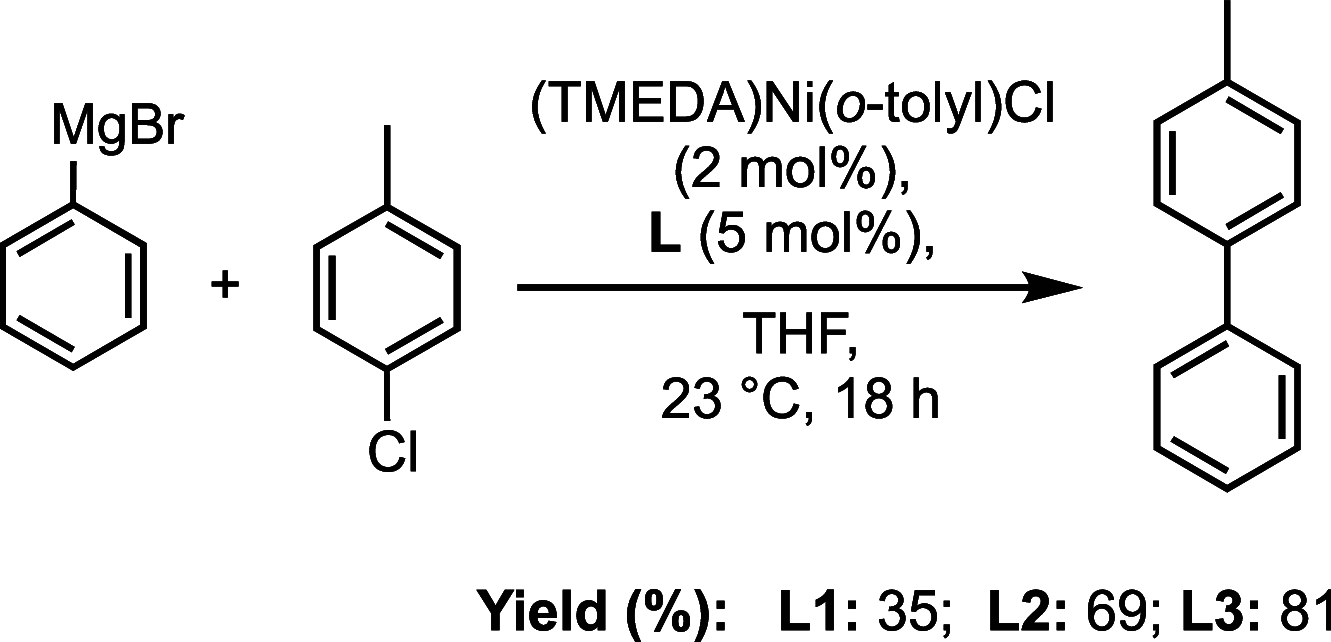
Understanding the net donor and electronic properties of pyrrole-based phosphines is critical for guiding their use as ligands. In this study, we compare two isomeric 1- and 2-(diphenylphosphino)methylpyrroles (L1 and L2, respectively) to determine the degree to which N-(phosphino)pyrroles are distinct from aryl- and 2-pyrrolyl phosphines. Ruthenium, rhodium, platinum, and gold complexes as well as selenide derivatives of these ligands are examined using NMR and IR spectroscopy, X-ray crystallography, and cyclic voltammetry. Ligand L2 exhibits net donor properties similar to those of the o-tolyl analogue L3, while L1 shows attenuated electron donation ability. Additionally, a model nickel-catalyzed Kumada coupling reaction using these three ligands was investigated.
© 2023 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
-
- Moloy K. G.; Petersen J. L. N-Pyrrolyl Phosphines: An Unexploited Class of Phosphine Ligands with Exceptional .pi.-Acceptor Character. J. Am. Chem. Soc. 1995, 117, 7696–7710. 10.1021/ja00134a014. - DOI
-
- Jackstell R.; Klein H.; Beller M.; Wiese K.-D.; Röttger D. Synthesis of Pyrrolyl-Indolyl-and Carbazolylphosphanes and Their Catalytic Application as Ligands in the Hydroformylation of 2-Pentene. Eur. J. Org Chem. 2001, 2001, 3871–3877. 10.1002/1099-0690(200110)2001:20<3871::AID-EJOC3871>3.0.CO;2-V. - DOI
-
- Alsalahi W.; Grzybek R.; Trzeciak A. M. N-Pyrrolylphosphines as Ligands for Highly Regioselective Rhodium-Catalyzed 1-Butene Hydroformylation: Effect of Water on the Reaction Selectivity. Catal. Sci. Technol. 2017, 7, 3097–3103. 10.1039/C7CY00200A. - DOI
LinkOut - more resources
Full Text Sources
