3D printed bioabsorbable composite scaffolds of poly (lactic acid)-tricalcium phosphate-ceria with osteogenic property for bone regeneration
- PMID: 38213985
- PMCID: PMC10776431
- DOI: 10.1016/j.bbiosy.2023.100086
3D printed bioabsorbable composite scaffolds of poly (lactic acid)-tricalcium phosphate-ceria with osteogenic property for bone regeneration
Abstract
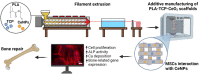
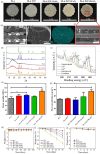
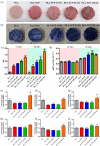
The fabrication of customized implants by additive manufacturing has allowed continued development of the personalized medicine field. Herein, a 3D-printed bioabsorbable poly (lactic acid) (PLA)- β-tricalcium phosphate (TCP) (10 wt %) composite has been modified with CeO2 nanoparticles (CeNPs) (1, 5 and 10 wt %) for bone repair. The filaments were prepared by melt extrusion and used to print porous scaffolds. The nanocomposite scaffolds possessed precise structure with fine print resolution, a homogenous distribution of TCP and CeNP components, and mechanical properties appropriate for bone tissue engineering applications. Cell proliferation assays using osteoblast cultures confirmed the cytocompatibility of the composites. In addition, the presence of CeNPs enhanced the proliferation and differentiation of mesenchymal stem cells; thereby, increasing alkaline phosphatase (ALP) activity, calcium deposition and bone-related gene expression. Results from this study have shown that the 3D printed PLA-TCP-10%CeO2 composite scaffold could be used as an alternative polymeric implant for bone tissue engineering applications: avoiding additional/revision surgeries and accelerating the regenerative process.
Keywords: Additive manufacturing; Biomaterial; Bone tissue engineering; Ceria nanoparticle; Polymer-matrix composites.
© 2023 The Authors. Published by Elsevier Ltd.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



References
-
- Koons G.L., Diba M., Mikos A.G. Materials design for bone-tissue engineering. Nat Rev Mater. 2020;5:584–603.
-
- Harb S.V., Uvida M.C., Trentin A., Lobo A.O., Webster T.J., Pulcinelli S.H., Santilli C.V., Hammer P. PMMA-silica nanocomposite coating: effective corrosion protection and biocompatibility for a Ti6Al4V alloy. Mater Sci Eng C. 2020;110 - PubMed
LinkOut - more resources
Full Text Sources
