Risk of T2 lesions when discontinuing fingolimod: a nationwide predictive and comparative study
- PMID: 38214014
- PMCID: PMC10783644
- DOI: 10.1093/braincomms/fcad358
Risk of T2 lesions when discontinuing fingolimod: a nationwide predictive and comparative study
Abstract
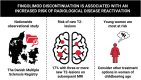
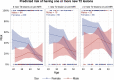
Fingolimod is a frequently used disease-modifying therapy in relapsing-remitting multiple sclerosis. However, case reports and small observational studies indicate a highly increased risk of disease reactivation after discontinuation. We aimed to investigate the risk of radiological disease reactivation in patients discontinuing fingolimod. We performed a nationwide cohort study in Denmark, including patients who discontinued fingolimod between January 2014 and January 2023. Eligibility was a diagnosis with relapsing-remitting multiple sclerosis and two MRIs performed respectively within 1 year before and after discontinuing fingolimod. The included patients were compared with those discontinuing dimethyl fumarate with the same eligibility criteria in an unadjusted and matched propensity score analysis. Matching was done on age, sex, Expanded Disability Status Scale, MRI data, cause for treatment discontinuation, treatment duration and relapse rate. The main outcome was the presence of new T2 lesions on the first MRI after treatment discontinuation. To identify high-risk patients among those discontinuing fingolimod, we made a predictive model assessing risk factors for obtaining new T2 lesions. Of 1324 patients discontinuing fingolimod in the study period, 752 were eligible for inclusion [mean age (standard deviation), years, 41 (10); 552 females (73%); median Expanded Disability Status Scale (Q1-Q3), 2.5 (2.0-3.5); mean disease duration (standard deviation), years, 12 (8)]. Of 2044 patients discontinuing dimethyl fumarate in the study period, 957 were eligible for inclusion, presenting similar baseline characteristics. Among patients discontinuing fingolimod, 127 (17%) had 1-2 new T2 lesions, and 124 (17%) had ≥3 new T2 lesions compared with 114 (12%) and 45 (5%), respectively, for those discontinuing dimethyl fumarate, corresponding to odds ratios (95% confidence interval) of 1.8 (1.3-2.3) and 4.4 (3.1-6.3). The predictive model, including 509 of the 752 patients discontinuing fingolimod, showed a highly increased risk of new T2 lesions among those with disease activity during fingolimod treatment and among females under 40 years. This nationwide study suggests that discontinuing fingolimod in some cases carries a risk of developing new T2 lesions, emphasizing the importance of clinical awareness. If feasible, clinicians should prioritize the prompt initiation of new disease-modifying therapies, particularly among young females.
Keywords: cohort study; disease-modifying therapy; multiple sclerosis; safety; treatment discontinuation.
© The Author(s) 2024. Published by Oxford University Press on behalf of the Guarantors of Brain.
Conflict of interest statement
M.F.W.-H. has served on the scientific advisory board for Sanofi and has received honoraria for lecturing for Novartis and Sanofi. R.P.H. has served on the scientific advisory board for Novartis and received honoraria for lecturing for Novartis and Sanofi. A.H. has served on the scientific advisory board for Merck and received honoraria for lecturing for Biogen and Merck. A.R.L. reports no competing interests. M.M. has served on the scientific advisory board for Biogen, Sanofi, Roche, Novartis, Merck and AbbVie, has received honoraria for lecturing from Biogen, Merck, Novartis, Sanofi and Genzyme and has received research support and support for congress participation from Biogen, Genzyme, Teva, Roche, Merck and Novartis.
Figures
References
-
- European Medicines Agency . Gilenya—EMEA/H/C/002202—IA/0080. Published online 2022:28. https://www.ema.europa.eu/en/documents/product-information/gilenya-epar-...
-
- Cavone L, Felici R, Lapucci A, et al. Dysregulation of sphingosine 1 phosphate receptor-1 (S1P1) signaling and regulatory lymphocyte-dependent immunosuppression in a model of post-fingolimod MS rebound. Brain Behav Immun. 2015;50:78–86. - PubMed
-
- Berger B, Baumgartner A, Rauer S, et al. Severe disease reactivation in four patients with relapsing–remitting multiple sclerosis after fingolimod cessation. J Neuroimmunol. 2015;282:118–122. - PubMed
LinkOut - more resources
Full Text Sources
